Student Essays and Reports
The fullerene family and fullerene-based materials
Pekka Aittala
Fullerenes are a family of a series of hollow molecules that
consists of carbon only. Fullerenes are the third well-defined
allotrope of carbon, the other two being diamond and graphite.
Fullerenes can be divided into three classes on the basis of the
shape. Spherical and cylinder-shaped fullerenes are also known as
'buckyballs' and 'nanotubes', respectively (see Figure 1). The
third class of fullerenes has a shape of an ellipsoid. [1] In many
occasions the buckyballs are called simply as fullerenes.
Buckyballs
Fullerenes were officially discovered in 1985 by H.W. Kroto,
R.E. Smalley, and R.F. Curl Jr. whose paper reported the discovery
of the spherical carbon molecule consisting of 60 atoms, i.e.
C60. [2] The new large carbon molecule was named
buckminsterfullerene after the American architect R. Buckminster
Fuller who designed a geodesic dome that has exactly the same
shape with C60. It should be noted, however, that E.
Osawa predicted the presence of the spherical fullerene
C60 in 1970's. Because the results were published in a
Japanese journal, respectively, the hypothesis did not reach the
Western civilization.

Figure 1. Structures of a) Buckminster fullerene
C60 [3], b) carbon nanotube [4], and c) carbon nanobud [5].
Structure and properties
It follows from Euler's theorem that there are always exactly
12 pentagons in buckyballs, whereas the number of hexagons
determines the size of the fullerene. Because the number of
pentagons is constant, the smallest possible buckyball is
C20. Moreover, fullerenes with up to 100 carbon atoms
are common. However, the C60, which has a diameter of
ca. seven angstroms, is the most common and therefore most studied
fullerene. Even though all the carbons in the fullerene are
conjugated, the fullerene molecule is not aromatic. Consequently,
the electrons of the hexagonal rings of the fullerene do not
delocalize over the whole molecule. The double bonds of the
fullerene are not equal but the hexagon-hexagon bonds are shorter
than the hexagon-pentagon bonds. [6]
Fullerenes are stable but not completely unreactive. The
characteristic fullerene reaction is electrophilic addition to
hexagon-hexagon double bonds. In the addition the sp2
-hybridized carbons are changed into sp3 -hybridized
ones. The change in hybridized orbitals decreases the bond angles
and the bonds have to bend less when the spherical structure is
formed and the fullerene becomes more stable. [6]
Fullerenes are hardly soluble to the polar solvents. However,
fullerenes are the only allotrope of carbon that can be dissolved
in a common solvent in a room temperature. The most common solvent
that dissolves fullerenes is benzene. Solutions of pure
C60 and C70 have deep purple and reddish
brown color, respectively. [7]
Applications
It is possible to trap a metal atom inside the fullerene cage.
These endohedral species are assigned as M@C60, in
which the M stands for metal. Also helium can be trapped inside
the fullerene cage by heating fullerene in helium vapor under high
pressure. [1] If the M is alkali metal, the structure of the
M@C60 is rather disordered. The bulk structure consists
of grains, i.e. small regions of a crystal structure. The size of
the grains may vary between tens of Angstroms to some microns. The
orientation of the individual grains may not have any correlation
at all. [8] The interaction between fullerene and alkali metals is
based on the high electron affinity of the fullerene and
electropositivity of the alkali metals.
Nowadays the development of organic solar cells is a hot topic.
An essential task in developing the solar cell is to find an
appropriate electron transfer pair. Porphyrin is an obvious
chromophore to be used in the electron transfer dyads because of
its occurrence in the photosynthetic reaction center in the
nature. The quinone acceptor involved in the reaction center is,
however, more often replaced with a fullerene. [9] The reason is
simple, e.g. fullerene C60 can accept up to six
electrons. [10] The high electron affinity of the fullerene is due
to its energetically low-lying triply degenerate lowest unoccupied
molecular orbitals (LUMOs). [11] Nowadays there are plenty of
investigations reporting porphyrin-fullerene dyads. An example of
such a dyad is presented in Figure 2.

Figure 2. An example of a porphyrin-fullerene
donor-acceptor dyad [9].
The poor water solubility of the fullerenes has restricted
their biological applications. There has been, however, several
attempts to try to use fullerenes e.g. in enzyme inhibition and
antiviral activity. Most of these attempts date back, close to the
discovery of the fullerenes, to the early 90's. [12] Although, the
solubility of the fullerenes can be enhanced by adding
surfactants, the carbon nanotubes are actually more promising than
buckyballs as it comes to applications.
Nanotubes
The nanotubes were officially discovered shortly after
buckyballs, i.e. in 1991, by Iijima Sumio [13]. However, the
history of the nanotubes is very similar to that of buckyballs.
The nanotubes vere actually found almost 40 years before the work
of Sumio as Radushkevich and Lukyanovich reported hollow graphitic
fibers in 1950's in a Russian journal.
Structure and properties
Nanotubes are
cylindrical fullerenes which have a diameter of few nanometers and
length of up to millimeters [14]. Straight (defectless) nanotubes
have only hexagonal rings in their structure. If nanotubes have
closed ends, they must contain also pentagonal rings (for the sake
of Euler's theorem). Nanotubes may also contain heptagonal rings,
which induce changes to the diameter of the nanotube. There are
basically two types of nanotubes, single-walled (SWNT) and
multi-walled nanotubes (MWNT). Double-walled nanotubes (DWNT) are
a typical example of MWNTs.
The nanotubes have only sp2 hybridized bonds and
they are actually bent sheets of graphine. This explains the
extraordinary strengths of carbon nanotubes. The nanotubes are
actually stronger than diamond since diamond structure has weaker
sp3 bonds. The properties of the nanotube depend how the graphite
sheet has been rolled into a tube and therefore the nanotubes have
been named on these basis. If integers n and m are
the number of unit vectors of a graphine structure, the different
types of nanotubes can be characterized by using these integers.
Figure 3 illustrates the 'zigzag' (n, 0), 'armchair'
(n,n) and 'chiral' (n,m) structures of
nanotubes.

Figure 3. Forming of the zigzag, armchair, and
chiral nanotube. [5]
The conductivity of an individual nanotube (n,m)
depends on the helical orientation of the hexagonal rings in the
walls of the nanotube i.e. on the n and m in a
following way. If n and m are equal (the armchair
structure), the nanotube is metallic and otherwise the nanotube is
a semiconductor. Metallic nanotubes can carry over 1000 times
higher electrical current densities than metals. Additionally,
electrons will transport only along the direction of the axis of
the nanotube. [15] There are almost limitless amount of possible
applications of the conducting nantoube bundles. Electronic flat
panel devises are just one example. [1] In addition to the
electron conductance, nanotubes are also clearly better heat
conductors than metals. [16]
Some applications
Owing to their unique properties, there are several potential
applications for carbon nanotubes. The electronic properties and
size of nanotubes allow several applications in electronics. These
include transistors that are capable for switching by using just
one electron [17], or ultra light and efficient coolers for
electronics [18]. The extreme strength / weight ratio of carbon
nanotubes make them potential materials for armors [19]. These are
just few examples of the diverse potential of the nanotubes.
Nanobuds
Carbon nanobuds are molecules in which the spherical fullerene
is covalently linked to the outer sidewall of a nanotube. Nanobuds
combine properties of the buckyballs and nanotubes. For example,
the conductivity of the nanobuds is similar to that of nanotubes,
but nanobuds are more reactant than nanotubes because the attached
buckyball. [20]
Summary
Two decades after the discovery of the fullerene family, the
third fell-defined allotrope of carbon, the scientists have
learned much about the fascinating properties of these molecules.
Thought the chemistry of the fullerenes is yet far from being
completely solved, it seems clear that these molecules have huge
potential for applications in several different fields of
science.
References
[1] H.W. Kroto, D.R.M. Walton, Fullerene. (2009). In Encyclopedia
Britannica. Retrieved August 7, 2009, from Encyclopedia Britannica
Online: http://search.eb.com/eb/article-234438
[2] H.W. Kroto, J.R. Heath, S.C. O'Brien, R.F. Curl, R.E. Smalley,
Nature 318 (1985) 162.
[3] Nature.com, retrieved August 8, 2009 from:
http://www.nature.com/nature/history/images/timeline/1980_6.jpg
[4] The International NanoScience community, retrieved August 8
2009 from:
http://api.ning.com/files/hhEvnlUQK-zEz08mtquoJu3yTOoEIMYLTvZl5lvQzSDUwyfgwwXIw6LxNXnKDbXK8Qge6Hu4L7V-7XHsuPFp9cs*prT1NOwv/tube_angled.jpg
[5] Wikipedia.com, retrieved August 8 2009 from:
http://commons.wikimedia.org/wiki/Category:Carbon_nanotube
[6] M. Prato, J. Mater. Chem. 1 (1997) 1097.
[7] V.N. Bezmelnitsyn, A.V. Eletskii, M.V. Okun, Russian Academy
of Sciences, 41 (1998).
[8] K. Allen, F. Hellman, Phys. Rev. B 60 (1999) 11765.
[9] Q. Xie, E. Perez-Cordero, L. Echegoyen, J. Am. Chem. Soc. 114
(1992) 3978.
[10] R.C. Haddon, L.E. Brus, K. Raghavachari, Chem. Phys. Lett.
125 (1986) 459.
[11] P.A. Liddell, J.P. Sumida, A.N. MacPherson, L. Noss, G.R.
Seely, K.N. Clark, A.L. Moore, T.A. Moore, D. Gust, Photochem.
Photobiol. 60 (1994) 537.
[12] A.W. Jensen, S.R. Wilson, D.I. Schuster, Bioorg. Medic. Chem.
4 (1996) 767.
[13] S. Iijima, Nature, 354 (1991) 56.
[14] L.X Zheng, M.J. O'Connell, S.K. Doorn, X.Z. Liao, Y.H. Zhao,
E.A. Akhadov, M.A. Hoffbauer, B.J. Roop, Q.X. Jia, R.C. Dye, D.E.
Peterson, S.M. Huang, J. Liu, Y.T. Zhu, Nature Materials 3 (2004)
673.
[15] S. Hong, S. Myung, Nature Nanotechnology 2 (2007) 207.
[16] E. Thostenson, C. Li, T.-W. Chou, Composites Science and
Technology 65 (2004) 491.
[17] Ch. Postma, T. Teepen, Z. Yao, M. Grifoni, C. Dekker, Science
293 (2001) 76.
[18] K. Kord's, G. T'th, P. Moilanen, M. Kumpum'ki, J. V'h'kangas,
A. Uusim'ki, R. Vajtai, P.M. Ajayan, Appl. Phys. Lett. 90 (2007)
123105.
[19] T. Ylidrim, O. G'lseren, C. Kilic, S. Ciraci, Phys. Rev. B 62
(2000) 12648.
[20] A.G. Nasibulin, P.V. Pikhitsa, H. Jiang, D.P. Brown, A.V.
Krashennikov, A.S. Anisimov, P. Queipo, A. Moisala, D. Gonzalez,
G. Lientsching, A. Hassanien, S.D. Shandakov, G. Lolli, D.E.
Resasco, M. Choi, D. Tomanek, E.I. Kauppinen, Nature
Nanotechnology 2 (2006) 156.

High pressure Raman spectroscopy of double wall carbon nanotubes
Abdul Waheed Anwar
Double wall carbon nanotubes are the simplest representation of
multiwall carbon nanotubes. Higher electrical conductivity then
single wall carbon nanotubes, existence of excitonic states and
shielding of inner wall by outer wall to maintain the purity makes
the double wall carbon nanotubes attractive for potential
applications in the field of electronics and composite materials.
In this essay we discuss the high pressure Raman spectroscopy of
double wall carbon nanotubes to explore the properties of the
walls of carbon nanotubes and the interaction between the walls of
carbon nanotubes.
Raman Spectroscopy
Raman spectroscopy has been very useful technique to
characterize the structural properties carbon nanotubes. When a
light wave, considered as a propagating oscillating dipole, passes
over a molecule, it can interact and distort the cloud of
electrons round the nuclei. As the oscillating dipole interacts
with the molecule as it passes, it causes the electrons to
polarize. The light is released immediately as scattered
radiation. This process differs from an absorption process as the
additional energy does not promote an electron to an excited state
of the static molecule, in addition that the radiation is
scattered as a sphere and not lost by energy transfer within the
molecule or emitted at a lower energy. When scattering occurs,
only a small fraction of the radiation energy is scattered.
Mainly, the frequency of the scattered electromagnetic radiation
is unchanged and the elastic scattered radiation gives rise to the
so-called Rayleigh scattering. However, a smaller number of
photons may scatter in-elastically; the interacting molecule can
be excited from the ground state to any vibrational state; if this
happens, the law of conservation of energy states that the
scattered photons have energy slightly different from the
frequency of the incident photons (Stokes scattering). It is also
possible that an excited molecule will return from its excited
state to its ground state and this process adds the energy of the
transition to an incident photon, resulting in the shortening of
the wavelengths of the scattered radiation (anti-Stokes
scattering).
Experimental Details
For high pressure experiments Diamond Anvil Cell (DAC) and
Renishaw Raman spectrometer was used with laser wavelength 633 nm.
The operation of the diamond anvil cell relies on the following
principle: Pressure = Force / Area Therefore high pressure can be
achieved by applying a moderate force on a small area, rather than
applying a large force on a large area. In order to prevent
deformation and even breakage of the anvils that apply the force,
they must be made from a very hard and virtually incompressible
material: Diamond. The standard DAC has been extensively used to
the range of 10 - 300 kbar. Before high pressure Raman
spectroscopy nanotubes were doped pressure transmitting medium and
then sonicated. Gas kits to be used in DAC for high pressure were
prepared. After that nanotubes were loaded in the DAC with ruby.
Ruby lines are used to measure the pressure. Ruby lines shifts
linearly with pressure.
Results and conclusion
Here the behavior of double walled carbon nanotubes with
sulphuric acid as pressure transmitting medium under pressure
through their higher energy first order Raman mode (GM) is
discussed.  Inner and outer
wall Raman shift with pressure. Using the differences in the GM
line-shape and shift, the behaviour of the double wall carbon
nanotubes prepared by different routes tubes under pressure is
examined. The results suggest a blue shift in these transitions
while increasing pressure. In addition, a variation in value of
the transition pressure, when loading the different samples under
same pressure range is observed. This variation was related to a
variation in the diameter of the resonant tubes and difference of
synthesis. The difference of slopes for inner and outer wall
indicates the difference of interaction between the inner and
outer tubes for different types of tubes. The difference of
pressure experienced by same sample with the variation of
concentration of sulphuric acid is also observed. This suggests
the difference of structuring of sulphuric acid around the
nanotubes when concentration is changed.
References
[1] P. Puech, A. Bassil, J. Gonzalez, Ch. Power, E. Flahaut, S.
Barrau, Ph. Demont, C. Lacabanne, E. Perez, and W. S.
Bacsa, Phys. Rev. B 72, 155436 (2005).
[2] Pascal Puech, Ahmad Ghandour, Andrei Sapelkin, Cyril Tinguely,
Emmanuel Flahaut, David J. Dunstan, and Wolfgang Bacsa,1Phys. Rev.
B 78, 045413 (2008)
[3] P. Puech, E. Flahaut, A. Bassil, T. Juffmann, F. Beuneu and W.
S. Bacsa, J. Raman Spectrosc. 2007; 38: 714720

Carbon nanotubes in solar cells and other energy applications
Muhammad Imran Asghar and Marina Zavodchikova
Introduction.
With the dawn of 21st century, increasing global energy needs
and the environmental pollution have made renewable energy sources
a hot topic for research. Breakthroughs in nanotechnology open up
the possibility of moving beyond our current alternatives for
energy supply by introducing technologies that are more efficient,
inexpensive, and environmentally friendly. The integration of
nanotechnology in energy has raised hopes among the researcher
that it would dramatically help to reach the target. Carbon
nanotubes are intensively being studied and employed in different
energy technologies to increase the efficiency and to decrease the
prices effectively. 85% of the todays energy needs are fulfilled
by fossil fuels which cause carbon emission in the atmosphere. So
the importance of clean energy generation have become more
important, specially the solar cells.
Carbon Nanotubes in Solar cells application.
There are several kinds of solar cells. The 3rd generation
solar cells are trying to produce cheaper electricity with
improved efficiency with the help of CNTs. Dye sensitized
nanostructured solar cells (DSCs) are photo electrochemical solar
cells, consisting of a photoelectrode, redox electrolyte and
counter electrode.

Fig 1: Structure of dye sensitized nanostructured solar cell [1]
Carbon nanotubes (CNTs) can be applied to the DSCs at
photoelectrode, counter electrode and in electrolyte. Transport of
electrons through the nanoporous film of TiO2 is sometimes
problematic due to grain boundaries and increase in recombination.
Due to the excellent electrical properties of CNTs (ballistic
electron transport), it facilitates the electron transport and
improves the short circuit current of the DSCs. The single wall
CNTs (SWCNTs) seem to be quite adherent to TiO2 particles and
cause TiO2 particles to aggregate. On the negative side there is
less adsorbed dye and SWCNTs are non-uniformally distributed.
There are some stability issues as well which need to be resolved
for the practical application of CNTs at photoelectrode.

Fig 2: CNTs at photoelectrode of DSCs [2]
Acid treated multiwall CNTs (MWCNTs) are successfully employed at the photoelectrode of DSCs at low temperature (sintering at 150oC for 4 hours).

Fig 3: Images of CNTs at photoelectrode of DSCs [3]
CNTs form gels with ionic liquids by grinding which improves
the transport properties in the electrolyte [4]. When MWNTs and
SWNTs are dispersed into ionic liquid electrolytes, an improvement
in fill factor and open circuit voltage is observed [5].
MWCNT counter electrodes were found to be more efficient and
stable than sputtered Pt and electro-deposited Pt counter
electrodes in a 5 day stability test at room temperature [6]. In
a short term i.e. 30 days stability test in dark at room
temperature for MWCNTs, a continuous decrease in short circuit
current density Isc was observed due to detachment of weakly
adheared CNTs from the FTO glass and deposition on photoanode side
[7]. The larger diameter CNTs show better performance [7]. A short
term stability of SWCNTs for 12 days at room temperature was
reported [8]. A composite film of grapheme (1%wt) and PEDOT-PSS
showed significantly improved performance as counter electrode on
ITO substrates [9].
CNTs in DSC components seem to improve the performance of the
DSCs. However in literature amounts / weight percentages are
announced carelessly. CNT incorporation and sorting is difficult,
and manipulation methods may need to be improved further. CNTs are
still rather expensive (even though one does not need much in
composites), that is why synthesis methods in large quantities
should be developed.
Carbon Nanotubes in other energy applications:
Besides various promising application of CNTs in photovoltaics technology, there has also been advances in the use of CNTs to improve energy efficiency of wind turbines as an alternative renewable energy technology. For example, Eagle Windpower Oy, a Finnish technology company, has developed a solution to utilize CNTs and epoxy that binds nanotubes to make the windmill blades stronger and lighter (similar manufacturing technology was utilized in e.g. ski manufacture). In comparison with conventional glassfiber blades, the CNT-reinforced blades are 50% lighter, which allows the increase of the blade size, and extremely durable. Such small wind power stations are becoming more and more widespread nowadays as a simplified solution of locally produced wind power in houses, leisure homes and in agriculture [10].

Fig 4: Windmill with CNT-reinforced blades produced by Eagle Windpower Oy [11]
Furthermore, CNTs were utilized to fabricate high power
supercapacitors- electrical storage devices that are able to
deliver a large amount of energy in a short period of time. For
example, MIT researchers are on the way to commercialize a battery
based on capacitors that use CNTs for high surface area, allowing
fast charging and no degradation being advantageous over
traditional batteries that use a chemical reaction to produce
energy [16]. Recently, MIT researchers have demonstrated a
layer-by-layer method to fabricate pure, dense, thin films made of
uniform CNT layers able to carry and store a large amount of
charge, making them promising electrode materials [12].

Fig 5: Scanning-electron-microscope image of a film made up
of 30 layers of the nanotubes on a silicone substrate [12]
Figure 5 (B) is an example of a simple structure of a
supercapacitor using a dense CNT network fulfilling two main
functions in one single layer: an electrically conducting material
for the replacement of the metallic current collector, and the
active material to build up the electrochemical double layer. Such
a charge storage device is lightweight, low-cost and requires
simple room-temperature manufacturing technique [13].

Fig 6: Sketch (left) and picture (right) of a supercapacitor
using CNT networks for both the current collector and the
electrode. The CNT network and the separator (white strip) are
encapsulated in a PET container filled with electrolyte [13].
Recently, a new CNT-based solution was proposed to reduce the
costs of fuel cells where traditionally expensive platinum
catalysts are used, which also has other problems like reduced
efficiency due to surface-adhered carbon monoxide and properties
degradation with time. L. Dais group has published in Science a
novel idea of using an array of vertically aligned
nitrogen-containing carbon nanotubes (VA-NCNTs) produced by
pyrolysis of iron(II) phthalocyanine in either the presence or
absence of additional NH3 vapor, that could be used as an oxygen
reduction reaction (ORR) electrocatalysts even after the removal
of the residual Fe catalyst, showing a better long-term operation
stability than similar platinum-based electrodes [14].

Fig 7: (A) SEM image of the as-synthesized VA-NCNTs on a
quartz substrate. (B) TEM image of the electrochemically purified
vertically aligned nitrogen-containing carbon nanotubes
(VA-NCNTs). (C) Digital photograph of the VA-NCNT array after
having been transferred onto a PS-nonaligned CNT conductive
nano-composite film. Scale bars, 2 µm (A); 50 nm (B) [14].
Conclusions
According to European Union Photovoltaic Platform, 15% of the
worlds electricity will be from solar by the year 2050. It shows
that PV sector has a bright future. The integration of CNTs in
DSCs is drastically improving the performance of dye sensitized
solar cells. CNTs have also been employed successfully in other
renewable energy sources like wind mill, hydrogen storage etc.
Moreover, CNTs can have wide applications as alternative energy
storage media, utilizing fully their unique structural
characteristics.
References
[1] http://research.ncku.edu.tw/re/articles/e/20080516/5.html
[2] Kongkanand et al., Nano Lett. 7 (2006) 676680
[3] Lee et al., Sol. Energy Mater. Sol. Cells (2008) Sawatsuk et
al., Diamond & Relater Materials 18(2009), 524
[4] Science 300, 2072 (2003)
[5] Usui et al., J. Photochem. Photobiol. A: Chem. 164 (2004),
97101
[6] B.K. Koo, D.Y. Lee, H.J. Kim, W.J. Lee, J.S. Song, and H.J.
KimSeasoning effect of dye-sensitized solar cells with different
counter electrodes", J. Electroceram (2006) 17:79-82. DOI:
10.1007/s10832-006-9941-x
[7] T. N. Murakami, S. Ito, Q. wang, M.K. Nazeeruddin, T. Bessho,
I. Cesar, P. Liska, R. Humphry-Baker, P. Comte, P. Pechy, and M.
Grätzel,"Highly efficient dye sensitized solar cells based on
carbon black counter electrodes", Journal of The Electrochemical
Society, 153 (12) A2255-A2261 (2006).
[8] H. Zhu, j. Wei, K. wang, and D. Wu, Applications of carbon
materials in photovoltaic solar cells", Solar Energy Materials and
Solar Cells .Volume 93, Issue 9, September 2009, Pages 1461-1470.
doi:10.1016/j.solmat.2009.04.006
[9] P. Joshi, Y. Xie, M. Ropp, D. Galipeau, S. Bailey, and Q.
Qiao, Dye-sensitized solar cells based on low cost nanoscale
carbon/TiO2 composite counter electrode" Energy Environ. Sci.,
2009, 2, 426-429. DOI: 10.1039/b815947p
[10] [Advanced solution for harnessing light winds, Energy and
Enviro, TechKnowledge Ltd, 2009: www.energy-enviro.fi].
[11] [Nanotube Superbatteries, K. Bourzac, Technology review by
MIT, Jan. 2009]
[12] Bifunctional carbon nanotube networks for supercapacitors, M.
Kaempgen, J. Ma, G. Gruner, G. Wee and S. G. Mhaisalkar, Applied
Phys Lett, 90, 2007].
[13] Nitrogen-doped carbon nanotube arrays with high
electrocatalytic activity for oxygen reduction, K.Gong, F.Du,
Z.Xia, M.Durstock, L.Dai, Science, Vol. 323, No 5915, pp. 760-764,
2009.
[14] Wikipedia

Plasmon coupling in carbon nanotubes
Svitlana Baieva
Single-walled carbon nanotubes (CNT) are quasi-one-dimensional (1D) wires formed by graphene sheets rolled-up into cylinders with diameters 1-10nm and lengths 1-104 Āµm [1].
Each nanotube with particular (n,m) indices have characteristic electronic properties. The electronic density of states is a āfingerprintā of particular nanotube. In general carbon nanotubes can have metallic or semiconducting properties (the gap is either absent or it is present, see fig. 1) [2].

There are several mechanisms of light interaction with a nanotube: absorption, scattering, emission, diffraction and surface plasmon resonance (coherent oscillation of conductance electrons).
When light interacts with dielectric or semiconductor the creation of exciton (a bound state of electron and hole) occurs. The predominant part of optical experiments is done with semiconducting nanotubes. So, The theory was developed to research the possibility of exciton-plasmon coupling in semiconducting carbon nanotubes. The tube is modeled as infinitely thin, infinitely long, anisotropically conducting cylinder with its surface conductivity (
 is taken into account) obtained from the realistic band structure of a particular CNT. The total Hamiltonian of the coupled exciton-photon system on the nanotube surface is of the form: is taken into account) obtained from the realistic band structure of a particular CNT. The total Hamiltonian of the coupled exciton-photon system on the nanotube surface is of the form:
 (1) (1)
The first term represents free (medium assisted) electromagnetic (EM) field. It is possible to use the second quantized field Hamiltonian, which accounts the creation and annihilation of surface EM excitation of frequency
 at a point associated with carbon atom ( at a point associated with carbon atom (
 or n describes the position of the atom). The second term represents free exiton. The second quantized field Hamiltonian accounts the creation and annihilation of exiton with energy or n describes the position of the atom). The second term represents free exiton. The second quantized field Hamiltonian accounts the creation and annihilation of exiton with energy
 , here index f describes internal degrees of freedom of exiton. And the third term represents interaction between EM field and exiton. The physical meaning of the third term is following the exciton with the energy , here index f describes internal degrees of freedom of exiton. And the third term represents interaction between EM field and exiton. The physical meaning of the third term is following the exciton with the energy
 is excited through the electric dipole transition is excited through the electric dipole transition
 in the lattice site in the lattice site
 by the transversely (longitudinally) polarized surface EM modes. by the transversely (longitudinally) polarized surface EM modes.
This theory predicts that, first of all, the strong exciton-plasmon coupling in semiconducting carbon nanotubes must occur. In this energy region the dispersion curve of semiconducting carbon nanotube is continuous curve. But, when plasmon coupling occurs, the exciton dispersion curve exhibits Rabi-splitting (fig.2) [1]. If the dispersion curve changes so dramatically, the conductance also changes, when the plasmon coupling occurs. This can have variety of applications in the field of nanoelectronics and photonics. Such device as surface plasmon polariton controlled carbon nanotube transistor can be made.

References
[1] I.V. Bondarev, K. Tatur, L.M. Woods. Optics Communications. 282 (2009)
[2] R.Saito, M.Fujita, G.Dresselhaus, and M.S. Dresselhaus. Appl.Phys.Lett., vol.60 (1992).

Magnetic behavior of carbon nanosturtures: theoretical understanding and experimental controversies
Anastasiia Girka
At present time structures made up atoms of carbon that have nanometer scales became very interesting objects of investigation in spheres of condensed state physics and inorganic chemistry. Among these structures one can point out first of all, fullerenes, carbon nanotubes and thin multilayered nanostructures. They are interesting due to their unusual physical parameters (mechanical, electromagnetical and so on) that are absolutely absent in macro-material structures consistent of the same atoms. To explain the phenomena connected with displaying of such outstanding physical parameters one can apply laws of quantum physics, because changing ordinary properties of carbon starts after decreasing a size of examined structure until value ~ 100 nm. Carbon nanostructures are interesting both for studying fundamental properties of matter and for development of new informational and computers devices, for example sensing heads, elements of computer memory, etc.
As chemical element, carbon is a component of a lot of natural and synthetic materials thus, in macro-world it is well-known diamagnetic, in spite of that carbon nanostructures have unusual magnetic properties. Anybody knows that carbon in the form of graphite has enough high value of diamagnetic susceptibility χ~R2, here R is radius of circulated current ( for π-electrons, that cover 36 fundamental units of graphite, R≈ 0.78 nm). If an external constant magnetic field is oriented perpendicularly to graphites planes then the χ value for graphite is 25 times larger than the diamonds one. But for carbon nanotubes immersed into an external magnetic field that is oriented parallel to the tubes axes circulation of electrons take place along their surfaces thus R≈ 8 nm, therefore their diamagnetic susceptibility value is hundred times much as compared with value of this parameter for graphite.
Thin films made up of fullerens C60 illustrate untypical for macro-samples of carbon materials magnetic behavior in the Earth's magnetic field influenced by polarized light. Two samples of 30 nm and 250 nm thickness have demonstrated a significant changing of magnetic field intensity, in range from 3.4 to 12.9 nT, under the influence of polarization light.
Magnetic behavior of carbon nanostructures strongly depends upon impurity atoms either inserted into inner space of fullerens or embedded into a nanotubes carcass instead of some carbons atoms. In the capacity of such admixture one can mainly utilize atoms of irons group: Fe, Co and Ni. Development of air-stable single magnetic domain room temperature magnets is an important goal of nanotechnology. Recent important progress in stabilizing cobalt nanomagnets has been made through carbon encapsulation of the metal particles. The structures exhibit the magnetic hysteresis curve with a coercive force of H ≈ 200 Oe at low temperatures T~20 K and 260 Oe at 300 K. The temperature dependence of the magnetic susceptibility measured under zero-field cooling and 10 Oe field cooling conditions exhibits the behavior characteristic of a set of single magnetic domain nanomagnets in an amorphous carbon matrix. Single magnetic domain particles are
expected to show weak temperature dependence of the coercive force, while the saturation magnetization of the single domain magnets increases. Single domain magnets in carbon matrix are also expected to interact with each other through magnetic dipole-dipole interaction minimizing the global magnetization of the system at zero magnetic fields. In hetero-structured carbon nanotubes, partly filled by impurities on interface of carbon carcass and boron nitride segments a constant magnetic moment can acquire. Depending on the atomic arrangement, artificially formed super-lattices exhibit an itinerant ferromagnetic behavior. In some other nanostructures, presence of carbon radicals can lead to ferromagnetic behavior with a high Curie temperature. Unexpected giant magneto-conductance occurs in twisted nanotubes in presence of an external magnetic field.
Striking magnetic behavior has been found out in clusters of nanotubes, that compose general column with common outer surface (such structures at present time can be obtained during plasma discharges). If this nanocolumn is effected by an external magnetic field orielted perpendicularly to its axis of symmetry then magnetic flow of each tube becomes captured by the external field that induces electric currents which can circulate during some hours under the Helium temperatures. Even under the room temperatures this current is weakly damped: in some experiments value of eigen magnetic moment decreased half as much during two hours at T≈100°Ń. It means that electric current in nanostructures is characterized by abnormally high level of electric conductivity as compared with its value in conductors made up of macro materials. Theoretical investigation of this phenomenon proved that it can be explained only by application of laws of quantum physics, because through
nanocolumns with diameter 20 nm and length 10-5m under room temperatures one can transmit electric current with density j≈107A/cm2 and quantity of heating power will be only ~0.003 W. Therefore such high level of electric conductivity in nanocolumns exhibits its quantum character.
Concerning theoretical understanding of electromagnetic properties of nanostructures I can say that it keeps up with their experimental study barely. Its connected at first, with abstractness and complicated form of quantum physics laws; second, carrying out theoretical investigation of these properties one can be aware of multifunctional character of magnetic behaviors dependence both upon physical parameters of the studied nanostructure and upon manner of their production, presence of impurities and defects in carbons carcass; and at least, with technical difficulty of experiments, including preparation nanostructures. So in my mind, now the role of theoreticians in research into properties of nanostructures is auxiliary one, they only gather data, experimental facts, try to explain some dependences and experiments.

Electronic structure and properties of graphene
Armen Julukian
Graphene is made from carbon atoms arranged on a honeycomb crystall lattice made out of hexagons (see the Fig.1 [1]). One can call the graphene as a one-atom-thick planar sheet of sp2-bonded carbon atoms that are densely packed in a honeycomb structure.
Electronic structure of the graphene is based on the sp2 hybrids in which hybrid orbitals will form only σ bonds and one π (pi) bond is needed for the double bond between the carbons [2]. In this hybridization (mixing atomic orbitals) the 2s orbital is mixed with only two of the three available 2p orbitals [3] [4] [5]. The carbon-carbon bond length in graphene is approximately 0.142 nm. Graphene appears the basic structural element of some carbon allotropes including graphite, carbon nanotubes and fullerenes.
Concerning electronic properties of the graphene, it can be a semi-metal or zero-gap semiconductor. The dispersion relation between energy and wave number is linear for low energies near the six corners of the two-dimensional hexagonal Brillouin zone, leading to zero effective mass for electrons and holes [6] [7] yielding that at such low energies, electrons and holes near these six points, two of which are inequivalent, behave like relativistic particles described by the Dirac equation for spin 1/2 particles [8] [9] and the electrons and holes are called as DIrac fermions, and the corresponding six corners are Dirac points .
Experimentally it was observed that due to having unique electronic properties the graphene produces an unexpectedly high opacity for an atomic monolayer, with the absorbtion πα ≈ 2.3% of white light, where α is the fine-structure constant [10] [11]. Additionally, it has been shown that bandgap of graphene can be controlled from 0 to 0.25 eV by means of applying voltage to a dual-gate bilayer graphene field-effect transistor (FET) at room temperature [12]. Furthermore it was demonstrated that such unique absorption could become saturated ( non-linear behaviour called as Saturable absorption )when the input optical intensity above a threshold value (saturation fluency) giving opportunity for the graphene to be used in the wide application in ultrafast photonics because of ability of the graphene to be saturated under strong excitation from visible to near-infrared region due to the universal optical absorption and zero band gap. Also, the graphene can be utilized in spintronics as ideal material due to small spin-orbit interaction and near absence of nuclear magnetic moments in carbon, as well as electrical spin-current injection and detection in graphene was observed up to room temperature [13] [14] leading to such phenomena as spin coherence length above 1 micron at room temperature [15], and control of the spin current polarity with an electrical gate at low temperature [16].
Thermal conduction of the graphene is phonon-dominated [17]. Only in the case of a gated graphene strip the electronic contribution dominates over the phonon contribution at low temperatures when applying bias causing a Fermi Energy shift lareger than kT [18]. Despite its 2-D nature, graphene has 3 acoustic phonon modes. The two in-plane modes have a linear dispersion relation, whereas the out of plane mode has a quadratic dispersion relation. And the ballistic thermal conductance of graphene is isotropic [19].
Graphene is the strongest material ever tested [20] and thus, the graphine is very strong and rigid. For this reason the graphene can be utilized for NEMS applications such as pressure sensors, and resonators [21].
References:
[1] http://www.newworldencyclopedia.org/entry/Graphene
[2] J. P. Clayden, N. Greeves, S. G. Warren, P. D. Wothers (2000), Organic Chemistry (1st ed.), Oxford: Oxford University Press, p. 105, ISBN 978-0-19-8503 46-0
[3] Organic Chemistry, Third Edition Marye Anne Fox James K. Whitesell 2003 ISBN 978-0-7637-3586-9
[4] Organic Chemistry 3rd Ed. 2001 Paula Yurkanis Bruice ISBN 0-13-017858-6
[5] L. Pauling, J. Am. Chem. Soc. 53 (1931), 1367
[6] P. R. Wallace (1947). Physical Review The Band Theory of Graphite. 71. p. 622-634. http://link.aps.org/doi/10.1103/PhysRev.71.622 Physical Review.
[7] J.-C. Charlier, P.C. Eklund, J. Zhu, and A.C. Ferrari (2008). "Electron and Phonon Properties of Graphene: Their Relationship with Carbon Nanotubes". from Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties and Applications, Ed. A. Jorio, G. Dresselhaus, and M.S. Dresselhaus. Berlin/Heidelberg: Springer-Verlag.
[8] Semenoff, G. W. (1984). "Condensed-Matter Simulation of a Three-Dimensional Anomaly". Physical Review Letters 53: 5449. http://prola.aps.org/abstract/PRL/v53/i26/p2449_1.
[9] Avouris, P., Chen, Z., and Perebeinos, V. (2007). "Carbon-based electronics". Nature Nanotechnology 2: 605. http://www.nature.com/nnano/journal/v2/n10/abs/nnano.2007.300.html.
[10] Kuzmenko, A. B.; van Heumen, E.; Carbone, F.; van der Marel, D. (2008). "Universal infrared conductance of graphite". Phys. Rev. Lett. 100: 117401. doi:10.1103/PhysRevLett.100.117401.
[11] Nair, R. R.; Blake, P.; Grigorenko, A. N.; Novoselov, K. S.; Booth, T. J.; Stauber, T.; Peres, N. M. R.; Geim, A. K. (2008). "Fine Structure Constant Defines Visual Transparency of Graphene". Science 320: 1308. doi:10.1126/science.1156965. PMID 18388259. http://onnes.ph.man.ac.uk/nano/Publications/Science_2008fsc.pdf.
[12] Y. Zhang et al. (11 June 2009). "Direct observation of a widely tunable bandgap in bilayer graphene". Nature 459: 820-823. doi:10.1038/nature08105.
[13] Cho, Sungjae; Yung-Fu Chen, and Michael S. Fuhrer (2007). "Gate-tunable Graphene Spin Valve". Applied Physics Letters 91: 123105. doi:10.1063/1.2784934.
[14] Ohishi, Megumi; et al. (2007). "Spin Injection into a Graphene Thin Film at Room Temperature". Jpn. J. Appl. Phys. 46: L605-L607. doi:10.1143/JJAP.46.L605.
[15] Tombros, Nikolaos; et al. (2007). "Electronic spin transport and spin precession in single graphene layers at room temperature" (PDF). Nature 448: 571-575. doi:10.1038/nature06037.
[16] Cho, Sungjae; Yung-Fu Chen, and Michael S. Fuhrer (2007). "Gate-tunable Graphene Spin Valve". Applied Physics Letters 91: 123105. doi:10.1063/1.2784934.
[17] Balandin, A.A., Ghosh, S., Bao, W., Calizo, I., Teweldebrahn, D., Miao, F., and Lau, C.N. (2008). "Superior Thermal Conductivity of Single-Layer Graphene". Nano Letters ASAP. doi:10.1021/nl0731872.
[18] Saito, K., Nakamura, J., and Natori, A. (2007). "Ballistic thermal conductance of a graphene sheet". Physical Review B 76: 115409. doi:10.1103/PhysRevB.76.115409. http://link.aps.org/doi/10.1103/PhysRevB.76.115409.
[19] Saito, K., Nakamura, J., and Natori, A. (2007). "Ballistic thermal conductance of a graphene sheet". Physical Review B 76: 115409. doi:10.1103/PhysRevB.76.115409. http://link.aps.org/doi/10.1103/PhysRevB.76.115409.
[20] C. Lee et al. (2008). "Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene". Science 321: 385. doi:10.1126/science.1157996. http://www.sciencemag.org/cgi/content/abstract/321/5887/385. Lay summary.
[21] Frank, I. W., Tanenbaum, D. M., Van Der Zande, A.M., and McEuen, P. L. (2007). "Mechanical properties of suspended graphene sheets". J. Vac. Sci. Technol. B 25: 2558-2561. http://www.lassp.cornell.edu/lassp_data/mceuen/homepage/Publications/JVSTB_Pushing_Graphene.pdf.

Quantum transport in carbon nanotubes and graphene
nanoribbons
Manana Koberidze
Carbon nanotubes exhibit unusual physical and electronic properties what makes them extremely prospective for various applications in nanoscience, especially in nanotechnology. They can be metallic or semiconducting depending on their topology at the same time being mechanically strong - 100 times stronger though six times lighter than steel. All these properties together with high mobility of carriers in nanotube allow their utilization in nanocircuits as nanowires as well as building blocks in nano logic devices.
At the scale where the sizes (for example, the size of the wire) become small compared to the characteristic length of the electron motion, classical physics is not valid any more to describe physical phenomena. Instead, quantum effects appear. Consequently, electronic transport of nanotubes is also governed by the laws of quantum physics:In bulk materials according to Ohms law resistance depends on the material of the sample and its geometry: R = ρ L / A, where ρ is resistivity depending on the material only, L the length of the piece of material and A - its cross sectional area. Unlikely, in nanotubes, ρ does depend on L through quantum effects which will be described below.
Typical construction for observing quantum transport in nanotubes is two electrodes connected by a nanonwire (nanotube). Two main regimes of transports can be met in nanotubes: diffusive and ballistic, depending on their type, whether they are single-walled metallic (SWM), single-walled semiconducting (SWS) or multiwalled (MW): Ballistic transport is encountered in SWM and MW carbon nanotubes. As for diffusive transport, it can be met in all the three types.
Diffusive transport
In diffusive regime electron undergoes (multiple) reflections caused by elastic scattering from impurities, therefore, electron wavefunction changes during electron motion. In this case mean free path (λ) is considerably smaller than width (W) and the length of the wire (L): λ << W,L, where λ=vFτ. Here, vF is the Fermi velocity and τ is the scattering relaxation time.
Ballistic transport:
Ballistic transport is the case of an ideal conduction i.e. almost without scattering. As a result, the direction of the electron motion and consequently, its wavefunction remains unchanged. In this case λ >> W,L.
An amazing phenomena takes place in ballistic conductors at very low temperatures for the samples with small number of channels: contact conductance (as a result, resistance) is independent of material and size of the specimen, even more, is quantized in units of e2/h [1], each 1D channel conducts exactly 2e2/h kΩ. In case of multiple channels conductance is proportional to the number of channels determined by Landauer formula : G=(2e2/h)MT, M-number of channels, T transmission probability for the channel placed between two electrodes. The value of h/e2 = 25kΩ is called the quantum of resistance. It must be emphasized that G is due to the contacts which is linked by the nanowire and not due to the wire itself.
Graphene nanoribbons (GNR) are unrolled sheets of single-walled carbon nanotubes. Like other carbon nanostructures they exhibit interesting electronic properties and is seen as an alternative to silicon in nanoelectronics
Several studies using tight binding method have predicted that armchair GNRs can be either metallic or semiconducting depending on their widths [2-8]whereas ribbons with zigzag shaped edges are metallic [2-12].However, calculations through Density Functional Theory within Local Density approximation show that armchair nanoribbons can be semiconducting [13].
Experiments have shown that graphene nanoribbons have an energy gap near the charge neutrality point which decreases with increasing the width of the nanoribbon [14]. Explanation to the existance of an energy gap in nanoribbons with disordered edges has been suggested through Coulumb blockade [15] phenomenon, which arises due to the roughness at the edges of nanoribbons . Theoretical studies have shown that transport effects such as Coulomb blockade or a mobility
gap caused by edge disorder [16,17] may affect the accuracy of bandgaps measured under transport conditions and explain the independence of energy gap and crystallographic orientation. However, latest experimental studies have revealed that the crystallographic orientation of the graphene edges
significantly influences the electronic properties of nanoribbons [18].
It is notable that changing external conditions like doping, defects, chemical functionalization also may affect electronic properties of carbon nanostructures what can be used for tuning transport processes.
References
[1] B.J. van Wees et al., Phys.Rev.Lett (1988)
[2] M. Fujita, K. Wakabayashi, K. Nakada, and K. Kusakabe,
J. Phys. Soc. Jpn. (1996).
[3] K. Nakada, M. Fujita, G. Dresselhaus, and M. S.
Dresselhaus, Phys. Rev. (1996).
[4] K. Wakabayashi, M. Fujita, H. Ajiki, and M. Sigrist, Phys.
Rev. (1999).
[5] M. Ezawa, Phys. Rev. (2006).
[6] L. Brey and H. A. Fertig, Phys. Rev. (2006).
[7] K.-I. Sasaki, S. Murakami, and R. Saito, J. Phys. Soc. Jpn. (2006).
[8] D. A. Abanin, P. A. Lee, and L. S. Levitov, Phys. Rev. Lett. (2006).
[9] S. Okada and A. Oshiyama, Phys. Rev. Lett. (2001).
[10] H. Lee et al., Phys. Rev. (2005).
[11] Y.-W. Son, M. L. Cohen, and S. G. Louie, Nature (London) (2006).
[12] Y. Miyamoto, K. Nakada, and M. Fujita, Phys. Rev. (1999)
[13] V. Barone, O. Hod, and G.E. Scuseria, Nano Lett. (2006)
[14] M.Y. Han, B. Özyilmaz, Y. Zhang, and P. Kim, Phys. Rev. Lett. (2007)
[15] F. Sols, F. Guinea, and A. H. Castro Neto, Phys. Rev. Lett. (2007).
[16] D.Querlioz, et al. Appl. Phys. Lett. (2008).
[17] D.Gunlycke, D. A. Areshkin, and C.T. White, Appl. Phys. Lett. (2007).
[18] Kyle A. Ritter and JosephW. Lyding, Nature Materials (2009)

Optical response of carbon nanostructures (nanotubes, fullerenes, graphene)
Aleksandr Kondrashin
Carbon-based nanostructures are promising candidates for sensors and components in nanoscale circuitry [1,2]. Yet even with novel experimental geometries [3] and high-resolution scanning-probe techniques [4], transport measurements of individual molecules continues to be exceedingly difficult. Additionally, while recent measurements of the optical properties of single-walled carbon nanotubes (SWCNTs) have generated significant new insight [5,6], they have also raised major new questions about the role of many-electron effects in their absorption and luminescence spectra.
Optical response of nanotube
Stretching a carbon nanotube composite like taffy, researchers at the National Institute of Standards and Technology (NIST) and the Rochester Institute of Technology (RIT) have made some of the first measurements1 of how single-walled carbon nanotubes (SWNTs) both scatter and absorb polarized light, a key optical and electronic property. Recent research on the optics of SWNTs has focused on the behavior of excitons" the pairing of a negatively charged electron with the positively charged hole" that it leaves behind when it gets excited by a photon into a higher energy state. An important optical characteristic is how excitons in SWNTs impact the way the nanotubes absorb and scatter light.For example, how easy is it for the incident light to deform an exciton to create positive and negative poles? Theory says it should be significantly harder to do in a nearly one-dimensional nanotube than in a bulk semiconductor, where nearby electrons and holes reduce the amount of energy required. Measuring that is difficult because the effect depends on the orientation of the nanotubes, and theyre hard to line up neatly. The NIST/RIT team solved the problem elegantly by wrapping SWNTs with DNA to keep them from clumping together, and dispersing them in a polymer. When they heated the polymer and stretched it in one direction, the nanotubes aligned like sugar crystals lining up in pulled taffy, making the optical measurements possible. The team obtained the first experimental verification of the full optical response of individual semiconducting SWNTs, finding good agreement with theory.
In (8) was showed two new developments for treating the properties of nanostructured systems beyond their ground states. First, we outlined a new scattering-state approach for computing transport properties at finite bias voltage; then we discussed a many-electron Greens function technique for accurate computation of optical excitations. We considered two examples, four-atom carbon atomic wire nanojunctions and single-walled carbon nanotubes. For C4 nanojunctions, we predicted and explained the origin of the negative differential resistance and illustrated the important role the contact plays in determining the I V characteristics of molecular junctions. For carbon nanotubes, we showed that in order to explain qualitatively and quantitatively the optical absorption spectra of these carbon nanostructures, it is crucial to account formany-body (excitonic) effects beyond the conventional, one-particle level.
Optical response of fullerenes
In (9) was showed through the DFWM measurements that the fullerene series from C60 to C96 shows a large thirdorder nonlinear optical response at 0.532 ķm. The concentrations of fullerenes and the incident laser powers were found to be vitally important in the DFWM measurements and are therefore optimized. The second hyperpolarizabilities of these fullerenes were determined to be on the order of 10-31-10-30 esu, in which exists a trend that higher fullerenes show larger ē1111. The reasons for the trend were discussed based on the model of a free-electron gas confined in a spherical shell and the distortion-enhanced and resonance-enhanced third-order optical nonlinearity.
It is well known that nanophotonics problems show the new ways to study organic materials doped with fullerenes. A fullerene introduction in these materials is widely used due to high electron affinity of fullerenes (2.62.7 eV) that allows the intramolecular donoracceptor interaction to be reinforced. As it has been demonstrated in papers [10-11] for different donoracceptor structures, electron transfers not to the intramolecular acceptor fragment but to fullerene one. This complex is of a higher excited state absorption cross-section than the ground state one, it reveals unique spectral and dynamic characteristics
Optical response of graphene
The optical response of gapped graphene is of importance for several reasons. First, it is needed for an understanding of optoelectronic devices such as photodetectors and lightemitting devices. Second, optical spectroscopy might be applied for measurements of the magnitude of the energy gap.
Graphene is an appropriate system to examine because it is a two-dimensional semimetal [12-14], which has generated considerable interest because of the interesting physics associated with its unusual electronic structure and its promising device applications [15]. In particular, the optical properties of graphene display many intriguing features, such as a constant optical conductivity in the infrared-frequency regime, gatedependence optical absorbance, and a unique magneto-optical spectrum [16-18].
References
[1] C. Dekker, Phys. Today 52 (1999) 22.
[2] H. Park, J. Park, A.K.L. Lim, et al., Nature 407 (2000) 57.
[3] M.A. Reed, C. Zhou, C.J. Muller, et al., Science 278 (1997) 252.
[4] B.Q. Xu, N.J. Tao, Science 301 (2003) 1221.
[5] Z.M. Li, et al., Phys. Rev. Lett. 87 (2001) 127401.
[6] S.M. Bachilo, et al., Science 298 (2002) 2361.
[7] J.A. Fagan, J.R. Simpson, B.J. Landi, L.J. Richter, I.Mandelbaum, V. Bajpai, D.L. Ho, R. Raffaelle, A.R. Hight Walker, B.J. Bauer and E.K. Hobbie. Dielectric response of aligned semiconducting single-wall nanotubes. Physical Review Letters. 98, 147402 (2007).
[8] Electron transport and optical properties of carbon nanostructures from first principles J.B. Neaton et al. / Computer Physics Communications 169 (2005) 18
[9] Third-Order Nonlinear Optical Response of Fullerenes as a Function of the Carbon Cage Size (C60 to C96) at 0.532 ķ Huang et al. J. Phys. Chem. B 1998, 102, 61-66
[10] N.V. Kamanina, V.S. Vikhnin, A. Leyderman, A. Barrientos, Y. Cui, M. Vlasse, Opt. Spectrosc. 89 (2000) 369.
[11] N.V. Kamanina, Synth. Met. 127 (2002) 121.
[12] K. S. Novoselov et al., Science 306, 666 (2004).
[13] K. S. Novoselov et al., Nature (London) 438, 197 (2005).
[14] Y. Zhang, Y.-W. Tan, H. L. Stormer, P. Kim, Nature 438, 201 (2005).
[15] A. K. Geim and K. S. Novoselov, Nature Materials 6, 183 (2007); A. H. Castro Neto, et al., Rev. Mod. Phys. 81, 109 (2009), and references therein.
[16] F. Wang, et al., Science 320, 206 (2008).
[17] D. S. L. Abergel and Vladimir I. Fal'ko, Rev. B 75, 155430, (2007).
[18] V.P. Gusynin and S.G. Sharapov, Phys. Rev. B 73, 245411, (2006).

Carbon nanostructures beyond fullerenes, nanotubes and graphene: From nanobuds to foams to schwarzite to diamondoids
Heli Lehtivuori
Carbon nanobuds form a material which combines two allotropes of carbon: carbon nanotubes and fullerenes, shown in Fig. 1. In this material fullerenes are covalently bonded to the outer sidewalls of the underlying nanotube. Therefore, nanobuds exhibit proberties of both carbon nanotubes and fullerenes. For example, the electrical conductivity and the mechanical properties of the nanobuds are similar to those of corresponding nanotubes. However, because of the higher reactivity of the attached fullerene molecules, the hybrid material can be further functionalized through known fullerene chemistry. [1]
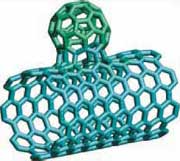
Figure 1. Carbon nanobud form a material which combines two
allotropes of carbon: carbon nanotube and fullerene [1]
Another interesting route to form low mass density all-carbon
structures, but with significantly higher mechanical stability,
are carbon foams, which may occur for example as honeycomb
graphite structures (see Fig. 2), but can also expose nanopores of
different geometrical shape. Their mechanical and electronic
properties will be highlighted in this contribution. [2]
 Figure 2. Structure of carbon foam (honeycomb
graphite) [3]
One type of negatively curved periodical nanostructures was
proposed by Terrones [4]. In the form of carbon zeolite-like
structures; he called them Schwarzites (see Fig. 3). They appear
to be some kind of cubic lattice, but they are two-dimensional
structures and thus their properties differ from those we have in
the ordinary crystalline materials. The carbon rings on the
negative curvature surfaces of schwarzite structures are expected
to include heptagons and/or octagons in addition to an
indeterminate number of hexagons. The carbon heptagons and
octagons introduce negative curvature into the schwarzite
surfaces. [4]

Figure 3. The four cells of Schwarzite [4]
In Fig. 3 diamondoid refers to variants of the carbon cage
molecule known as adamantane (C10H16), the smallest unit cage
structure of the diamond crystal lattice [5]. The unique structure
of adamantane is reflected in its highly unusual physical and
chemical properties. The adamantane comprises a small cage
structure. Because of this, adamantane and diamondoids in general
are commonly known as cage hydrocarbons. In a broader sense they
may be described as saturated, polycyclic, cage-like hydrocarbons
that are present in some reservoir fluids. [5]

Figure 4. The smallest unit cage structure of the diamond
crystal lattice is known as adamantane [5]
References
[1] Nasibulin, A. G. et al. Nature Nanotechnology 2, 156 (2007)
[2] Kuc, A. et al. Theor. Chem. Acc. 120, 543 (2008)
[3] Kuc, A. et al. Phys. Rev. B: Condens. Matter 74, (2006)
[4] Terrones H. et al., Phys. Rev. Lett. 84, 1716 (2000)
[5] Ramezani H. et al., J. Computl & Therorl Nanoscience 4, 96 (2007)

Strengths and limitations of theoretical approaches used to
study carbon nanostructures
Ville Petteri Mäkinen
Computers have become significantly more powerful since the
1940's. The raise of the computational power has made it possible
to use numerical simulations to describe different physical
phenomena from theoretical basis. Nowadays computational physics
has become an important branch of science, and also carbon
nanomaterials have been widely studied from theoretical basis.
Computer simulations have some aspects that are superior to
experiments. With computational approach one always knows exactly
the composition of the sample, and for example examining the
properties of perfect carbon nanotubes is simple. In experiments
there is always a possibility that some impurities or defects have
found their way in to the sample. Also, making slight
modifications to the sample (length of the tube) to see how it
affects the results is straightforward with computers. In
contrast, producing a specific impurity to the nanotube of certain
length and type in experiment is (at least practically)
impossible.
Collecting data from simulations is easy and it is possible to
investigate properties that are out of reach in experiments. For
example the electron density near the atoms of a fullerene inside
a carbon nanotube may help to understand why the nanoparticles
behave as they do. Therefore simulations can help to explain what
is going on in the experiment. Of course it is also very important
to look at the properties that can be measured in experiments in
order to make sure that the theoretical results agree with the
experimental ones.
The most sophisticated and used tool in studying electronic
structures from theoretical basis is density functional theory
(DFT) [1,2]. It gives very good results with a large variety of
systems. Unfortunately it is also computationally heavy method.
Therefore studies of nanomaterials with DFT are usually limited to
small nanoparticles or bulk calculations. The size limitation
causes problems when one wants to imitate an experiment in
computer, because in experiments e.g. carbon nanotubes are usually
longer than what is reasonable to simulate. Therefore one is often
required to build a small system that describes the real system as
well as possible with as few atoms as possible.
Fortunately carbon as a material is very convenient for
simulations. The electron wave functions of a carbon-only (or
hydrocarbon) materials can be very accurately described by using
atom-centered basis set consisting of only s- and p-type atomic
orbitals. Compared to more sophisticated methods (e.g. DFT with
plane wave implementation), this allows one to run simulations of
much larger systems and/or longer time periods (the speed compared
to DFT may easily be four orders of magnitude faster). These kinds
of methods are often called tight-binding approximations and there
is a huge number of different kinds of implementations, which
tells that the approximation works well in many situations.
Usually these kinds of methods require parametrization of
different element pairs. Each parametrization must be tested to
make sure that they produce physically sane interaction between
elements. The problem with parametrizations is that they are
usually tested against some reference systems. In principle there
is no guarantee that the parametrization gives reasonable results
for systems other than in the test set. However, this is a problem
in DFT also; pseudopotentials and certain hybrid functionals
contain parameters that are fitted to empirical data to give good
results for different test sets.
Even with these kinds of fast, more approximative methods
studying time evolution of large systems is still time consuming,
and studying for example how a certain reaction happens is nothing
but simple. As tempting it would be to start with some
configuration of atoms and see what happens as time goes by, one
is often limited to study total energies of different
configurations at some reaction path and conclude whether the
reaction might take place by following that path or not. Of course
there are infinite number of reaction paths and one may miss the
most important one.
Even with state-of-the-art methods there is always some
uncertainty in how much you can trust your results. With DFT,
being a ground state theory, all the properties connected to e.g.
electron excitations are more or less arbitrary. With further
approximations (i.e. a simpler theory) it becomes more and more
unclear which properties should come out right. For each system
one should think about what is the best tool to describe it. From
computational point of view the simplest is usually the fastest,
but it may not cover all the crucial physical phenomena required
to describe the system well.
References
[1] P. Hohenberg and W. Kohn, Inhomogenous Electron Gas, Phys.
Rev. 1964 volume 136, number 3B
[2] W. Kohn and L. J. Sham, Self-consistent equations including
Exchange and correlation effects, Physical Review 1965, volume
140, number 4A

Nature and effect of defects in carbon nanostructures
Tetyana Malykhina
Currently, many laboratories of the world are engaged
with the synthesis of carbon nanostructures. Most recent advances
in nanoscience and nanotechnology were made through the
fabrication and characterization of novel carbon-based
nanostructures such as the fullerenes, carbon nanotubes, and
graphenes. It is an indisputable fact that carbon has unique
chemical properties among all elements of the periodic table,
being essential to construct those nanomachines that existed in
the natural world before mans appearance on earth and work inside
the cells of all living organisms [1].
Fullerenes and carbon nanotubes can be formed by rolling up a
graphene sheet around a sphere and a cylinder, respectively.
Since this discovery, the carbon nanostructures attracted a lot of
attention of both experimentalists and theorists because of their
unique transport and mechanical properties as a
quasi-one-dimensional material with a nanometer-scale
dimension.Graphene shows extraordinary electronic transport
properties. A graphene sensor was able to detect the absorption of
a single gas molecule due to the high sensitivity of its
electrical resistance to local changes in charge carrier
concentration [1].
The interest of scientists to this new form of carbon is being
increased. In this regard, it is very interesting to perform a
brief analysis of defects in carbon nanostructures.
There are two main types of defects. The first one is a
vacancy obtained by extracting single atom. There are some known
facts about the structure and chemistry of the sidewall defect
sites that result from the removal of individual carbon atoms.
Several different defects are generated by removing one or two
carbon atoms from the side wall of a pristine zigzag nanotube.
When one carbon atom is removed from the nanotube side wall, three
carbons with dangling bonds are formed. A new bond can form
between two of these, generating a pentagon.
The second type of defect does not have missing atoms, but has
a failure in the atomic arrangement (topological defect), causing
two pairs of pentagons among the honeycomb network of hexagons.
The latter defect is called a Stone-Wales defect, named after the
two scientists who proposed it. This defect attaches to
the tube convex and concave surfaces. The tubes are curved
and swirl. This Stone-Wales transformation is thought to play an
important role during the growth of carbon nanostructures. Once
formed, the pentagon/heptagons could move along the structure,
creating either dislocation centers in regions of positive
(pentagons) or negative (heptagons) Gaussian curvature, which
ultimately lead to the closing of the nanostructure. The high
activation energy barrier of several electron volts for the bond
rotation in nanotubes would make the density of Stone-Wales
defects small in thermodynamic equilibrium. Still, these defects
should occur as metastable structures at room temperature, and can
be frozen in the nanotube body during the nonequilibrium growth
process. Note that other energetically comparable, but much more
visible defects, including isolated pentagons and heptagons
͑are often seen in transmission electron microscopy images,
since these defects induce bending and reshaping of the entire
nanotube structure at a relative small cost of elastic strain
energy [2].
Stone-Wales defects can be identified by the induction of a
defect-localized vibration by photo-excitation [2]. This technique
can also be used to monitor the formation of SW defects during
nanotube growth [2]. Stone-Wales defects can not be removed.
It was well known that nanotubes are still stable against
thermal excitation, even in the presence of these defects.
Nanotubes are not so stable against illumination. (This fact may
be obtained as a result of a molecular dynamics simulation.
However, using this simulation it is not easy to investigate
structural changes in materials induced by illumination).
However, nanotubes can repair of defects after illumination very
fast.
Internal defects and irradiation-induced defects in carbon
nanotubes influence on mechanical as well as electronic
properties of carbon nanotubes. Regardless of this fact, defects
in nanotubes can be useful, for example, for nanotube band
engineering, improving mechanical properties of macroscopic
nanotube-based materials and nanotube functionalization. Carbon
nanotubes and fullerenes have a lot of potential applications in
nanotechnology. Defects also influence the transport properties of
the nanotubes. Topological defects may occur in the as-grown
nanotubes, or they can be generated by several methods like
chemical treatment or irradiation with charged particles. Recent
experiments show that both electron and heavy ion irradiation can
modify the structure and dimensions of carbon nanotubes [3].
References
[1] E. W. S. Caetano, V. N. Freire, S. G. dos Santos, E. L.
Albuquerque, D. S. Galvao, and F. Sato, Defects in Graphene-Based
Twisted Nanoribbons: Structural, Electronic, and Optical
Properties, Langmuir, 2009, 25 (8), pp 47514759.
[2] Yoshiyuki Miyamoto, Angel Rubio, Savas Berber, Mina Yoon, and
David Tomanek. Spectroscopic characterization of Stone-Wales
defects in nanotubes. PHYSICAL REVIEW B 69, 121413 (R) 2004.
[3] Z. Osvįth, G. Vértesy, L. Tapasztó, F. Wéber, Z. E. Horvįth,
J. Gyulai, and L. P. Biró. Atomically resolved STM images of
carbon nanotube defects produced by Ar+ irradiation. PHYSICAL
REVIEW B 72, 045429 ͑2005.

Carbon nanostructures beyond fullerenes, nanotubes and
graphene: From nanobuds to foams to schwarzite to diamondoids
Vadym Markov
In this work I try to review different forms of carbon, mainly
from chemist's point of view. Main forms are linear carbons
– polyyne and polycumulene, glassy carbon, graphyte
compounds – such as alkali metals graphitides, graphene
oxide, graphite salts. Glassy carbon was discovered accidentally,
in 1960s [1]. It is obtained by temperature treatment of polymers,
not allowing graphitization [2]. X-ray diffraction analysis shows
presence of extremely distorted graphite-like structure. Very
small parts of graphite layers are placed chaotically.
Crystallites have size like 50x15A, compared with hundreds of
angstroms, typical for graphite. Inter-layers distance is 3.5 A
versus 3.35 A for graphyte. Such properties as density, porosity,
strength shows that sp3 - hybridized atoms are present.
Carbon foams are rather similar to glassy carbon in terms of
structure, but demonstrate very low density and they are
insulators. Carbon nanofoam was obtained first by Rode in 1999 [3]
by laser ablation of carbon in argon. Such environment leads to
high percent of sp3 bonds formed. Nanofoam consists of warped
graphite-like negative-curved sheets. Nanofoam has 300-400
g/m2 surface. sp2/sp3 bonds ratio
was studied by EELS method. sp2 fraction is near 0.9,
which is much lower comparing with glassy carbon.
Linear carbons are somewhat like limiting case of nanotube consists
of only one row of atoms. Obtaining of third carbon allotrope was
a great challenge for chemistry, because 3D form – diamond
and 2D – graphite was known very well. Organic chemistry
says that two variants of linear carbon are possible –
polycumulene with only double bonds and polyyne with alternating
single and triple bonds. They commonly called carbyne. Main way of
synthesis is gradual oxidation of C2H2
resulting a polyyne. Polycumulene was obtained through polymeric
acetylene-allene glycol with former reducing. These structures
were confirmed by ozonation analysis and IR spectroscopy. Linear
carbons forms hexagonal lattices with parallel threads of carbon.
Such structures are studied as object for developing organic
conductors.
Next class of carbon forms which I want to review is graphite
intercalation compounds. We cant say that this is carbon in strict
sense, but they keep sheet structure of graphite. Main classes of
graphite intercalation compounds are metal graphitides, such as
potassium graphitide KC8, graphite salts, such as
graphite sulfate and covalent compounds such as graphite oxide and
graphite acid.
Main feature of such compounds is layered structure. Intercalant
atoms or molecules create their own layers between graphite
layers. Different amount of intercalate can go into graphite.
Limit case is stage 1 intercalate. For potassium, it is
KC8, every other layer is potassium. Graphite layers
are bounded as AAAA. Potassium keeps reactivity and readily
oxidizes by air and so on.
Graphite sulfate can be obtained by direct syntesis or
electrochemically. H2SO4 molecules and
HSO4- ions are intercolated.
C24HSO4x2.5H2SO4 for
stage 1 and
C48HSO4x2.5H2SO4 are
known. These compounds are not stable on aur and absorb water with
decomposing easily.
Last class of compounds which I want to review is graphene oxide.
It is also called graphite oxide, graphitic oxide or graphitic
acid. Graphene oxide layers are about 1.1a0.2 nm thick. Scanning
tunnelling microscopy shows the presence of local regions where
oxygen atoms are arranged in a rectangular pattern with lattice
constant 0.27 nm x 0.41 nm The edges of each layer are terminated
with carboxyl and carbonyl groups. X-ray photoelectron
spectroscopy shows the presence of carbon atoms in the
non-oxygenated ring context (284.8 eV), in C-O (286.2 eV), in C=O
(287.8 eV) and in O-C=O (289.0 eV). [7] Such compounds are used
for graphene synthesis through reduction. Other nano applications
are also possible.
References
[1] J. C. Lewis, B. Redfern, and F. C. Cowlard, Solid-State
Electronics 6 (1963) 251.
[2] Cowlard, F. C.; Lewis, J. C. (1967). "Vitreous carbon —
A new form of carbon". Journal of Materials Science 2 (6):
507, 512.
[3] A.V. Rode, S.T. Hyde, E.G. Gamaly, R.G. Elliman, D.R..
McKenzie, S. Bulcock, Appl. Phys. A 69 (1999) S755.
[4] A. M. Sladkov, V. I. Kasatochkin, Yu. P. Kudryavtsev and V. V.
Korshak. Russian Chemical Bulletin, Volume 17, Number 12 /
December, 1968
[5] M.S. Dresselhaus, G. Dresselhaus. Intercalation compounds of
graphite. Advances in Physics, 2002, Vol. 51, No. 1, 1-186
[6] D. D. L. Chung Graphite review. Journal of Materials Science,
Volume 37, Number 8 / April, 2002
[7] D. Pandey and others (2008), Scanning probe microscopy study
of exfoliated oxidized graphene sheets. Surface Science, volume
602 issue 9, page 1607-1613

History, controversies and progress in hydrogen storage
in carbon nanostructures
Antti Ilmari Minkkinen
Scientists have been interest in using hydrogen as a fuel, but
the problem had been in how to store up hydrogen safely and
effectively [1]. Hydrogen has been tried to store up in chemical
storages, as a compressed hydrogen gas, as a liquid hydrogen, and
by gas-on-solid physical adsorption [2], but none of those have
been commercially profitable. Today nanoscientists think that
answer to this problem might be in nanotubes and in other
nanostructures.
One of the first reports about hydrogen adsorption on
high-surface-area carbon was reported by Kidnay and Hiza in 1967.
They found out that there is at least three ways to amount how
much gas is adsorbed to material surface: excess amount, total
amount and plotting amount of hydrogen against pressure. Excess
amount means 'the excess material present in the pores over that
which it would be present under the normal density at the
equilibrium pressure' [1]. In total amount all the matter that is
influenced by adsorption forces is calculated. It is also possible
to just plot the amount of hydrogen contained in the
adsorbent-filled container as a function of pressure. These three
ways are still used to report the amount of material adsorbed to
adsorbent. Kidnay and Hiza reported their maximum excess amount in
their experiment to be 20.2-g H2/kg carbon at 25 atm and 76 K,
corresponding to a gravimetric storage density of ~ 2.0 wt%, but
this profitable enough to store hydrogen. Storage density was too
low and keeping such a low temperature would be a problem in daily
use.
In 1980 Carpetis and Peschka suggested that hydrogen can be
stored inexpensively into activated carbon materials by adsorption
in very low temperatures. In their experiment they succeeded to
maximize hydrogen adsorption to ~5.2wt% at 41.5 atm and 65 K. But
the temperature was too low to store hydrogen commercially
profitable this way.
In the late 1980's and early 1990's research group of J.A.
Schwarz focused on fundamental aspects of hydrogen adsorption on
carbon nanomaterials. Their goal was that operating temperature of
the hydrogen adsorption could be increased. To achieve their goal
they investigated effects of surface acidity and metal
modifications. In their experiment they were able to maximize
hydrogen concentration only to 4.8 wt%, at a temperature of 87K
and a pressure of 59 atm. The group also carried out detailed
thermodynamic analyses and determined important parameters as the
isosteric heat of adsorption.
In 1999 Orimo et al. tried to increase the amount of hydrogen
storage into carbon sorbent by mechanically ball milling graphite
under a hydrogen atmosphere. Orimo et al. were able to get
hydrogen concentration to as high as 7.4 wt% after 80 hours of
milling. This was the first time when D.O.E.s (United States
Department of Energy) target values (6.5 wt% and 62-kg H2/m3) were
reached. Problem in this method was that reversibility of charging
and discharging was too unlikely.
Recent studies have shown that carbon nanofibers can store
hydrogen very effectively. Amount of hydrogen can be as high as 15
wt% in carbon nanofibers [2] which is over double of amount what
D.O.E. has set as the target value. Carbon nanofiber technique is
based on that hydrogen has a kinetic diameter of 0.289 nm which is
smaller than the space between two graphite sheets in carbon
nanofiber (~0.335-0.342 nm). When carbon nanofibers are exposed to
hydrogen under pressure of 120-130 atm at room temperature
hydrogen starts to slide between carbon layers. If the pressure is
lowered hydrogen starts to slide off between graphite sheets and
form hydrogen gas. So the hydrogen tank can be easily and
effectively charged and discharged without using very low
temperatures. Problem in this technique is that it needs a very
high pressure which can be problematic in daily life.
If carbon nanostructures will be main storage of hydrogen in
the future it still has to be polished. Hydrogen adsorption
capacity must be maximized at room temperature and under moderate
pressures. These tanks must be able to charge and discharge
rapidly and completely at near ambient conditions. It also will be
necessary to scale up the synthetic and purification techniques to
produce ideal hydrogen adsorption materials that can be done in
industrial-scale.
References
[1] Hydrogen storage using carbon adsorbents: past, present and
future, A.C. Dillion, M.J. Heben, National Renewable Laboratory,
Colorado, USA, 2000
http://www.springerlink.com/content/0hlb80b86ra3rbk4/fulltext.pdf
[2] Carbon nanofibers as a Hydrogen Storage Medium for Fuel Cell
Applications in the Transportation Sector, M.A. de la Casa-Lillo,
F. Lamari-Darkrim, D. Cazorla-Amoros, A. Linares-Solano, 2002
http://www.sjsu.edu/faculty/selvaduray/page/papers/mate115/robertzeches.pdf

Chemical functionalization of fullerenes and nanotubes,
and their applications
Pasi Moilanen
Fullerenes and carbon nanotubes have many interesting physical
properties. Their mechanical, electrical and structural
characteristics are exceptional. Nevertheless there are problems
to be solved to unleash their full potential for example in
biological applications. One issue is solubility. Carbon nanotubes
as prepared are insoluble. Fullerenes are only slightly better and
the solvents are not very friendly. With functionalization it is
possible to find new exciting application not possible with
pristine materials.
The conditions for functionalization are often quite harsh,
requiring quite high temperatures, long reaction times, high
pressure and using very reactive chemicals such as concentrated
sulphuric acid or nitrogen acid.
Fullerenes
Even though fullerenes were discovered earlier they fall
clearly behind carbon nanotubes in number of applications. One of
the most studied area is medical applications of fullerenes.
Fullerene derivatives have antiviral activity, they have
photodynamic activity, antioxidant activity, they can be used in
drug and gene delivery and there are also diagnostic applications
(1).
Since C60 is formed from pentagons and hexagons not
all double bonds are similar. There are bonds connecting pentagons
(6,6) and bonds connecting pentagons to hexagons (5,6). As these
bonds have different properties different methods can be used for
functionalization. (6,6) bonds can for example be used in
Diels-Alder reaction to add cyclic groups (2) 
Carbon nanotubes
There are three distinct ways to modify carbon nanotubes.
Covalently attaching functional groups onto pi-conjugated skeleton
of the tube, non-covalent adsorption and endohedral filling of the
tube.(3) The following is a examples of few possible ways to
functionalize carbon nanotubes and their applications.
CNTs can be hydrogenated via dissolved metal reduction with
lithium and methanol in liquid ammonia. With thermogravimetric and
mass spectroscopic analysis it was determined that CNT's have
stoichiometry of C11H. (4)
As an example of non-covalent adsorption is reversible
wrapping of SWCNT's with polymer, polyvinyl pyrrolidone. First
SWCNT's were dispersed in water with sodium dodecyl sulfate and
sonication. Then polymer was added and after 12h incubation at 50
C the resulting mixture was purified and as a result stable
solution of PVP wrapped SWCNT's was produced.

Some possible wrapping arrangements of PVP
Polymer composites are also an example of non-covalent
adsorption. Epoxy composites are maybe the most widely studied
composite type. One of the first carbon nanotube application
available for consumer markets was epoxy composite called
Hybtonite.
References
[1] Bonn et al. Int J Nanomedicine. 2007 December; 2(4):
639, 649
[2] Eguchi et al. Tetrahedron Volume 52, Issue 14, 1 April 1996,
Pages 4983-4994
[3]Prato et al. Chem. Rev., 2006, 106
(3), pp 1105, 1136
[4]Pekker et al. J. Phys. Chem. B, 2001, 105 (33), pp
7938–7943
[5]Smalley et al. Chemical Physics Letters Volume 342, Issues
3-4, 13 July 2001, Pages 265-271

Progress in the Synthesis and Formation of Graphene
Lauri Nykänen
Graphene is a sheet of carbon atoms in the form of two
dimensional hexagonal lattice. Materials such as graphite and
carbon nanotubes are composed of graphene sheets. It was presumed
that graphene doesn't exist in the free state until 2004 when
Novoselov et al. reported preparation of single-layer graphene by
applying Scotch tape on a piece of graphite [1]. The produced
graphene sheets proved to be of high quality and presented some
remarkable properties such as extremely high charge-carrier
mobilities and mechanical stiffness, which distinguishes it from
monoatomic metallic films.
The Scotch tape method (or mechanical exfoliation) produces
micron-size flakes suitable for laboratory research but the
scaling of the method for larger areas doesn't seem conceivable.
Since the discovery of free-standing graphene more methods have
been developed to manufacture graphene and they fall into three
categories: chemical vapour deposition (CVD) on metal surfaces
[2], epitaxial growth on insulator surfaces (such as SiC) in
ultrahigh vacuum [3] and the creation of colloidal suspensions
[4].
CVD method
In the CVD method a thin layer of nickel on a substrate is
exposed to a carbonaceous gas in a high temperature (1000ŗC).
Carbon atoms are formed on the surface and they migrate below the
surface to interstitial lattice sites. Then the nickel is cooled
down and the carbon atoms precipitate out of the nickel layer and
form graphene on the surface. Finally the graphene can be removed
from the nickel surface by gentle chemical etching and transferred
onto appropriate substrates. The CVD method seems to be the most
promising way of synthesizing graphenes of large area and high
quality. Three separate groups have reported fabrication of
single- and few-layer graphene with areas of square centimetres
manufactured with a CVD technique, and transferred to other
substrates [2, 5, 6]. Next step in the development of the CVD
method is to replace the polycrystalline substrates used by the
three groups with single-crystal nickel substrates. There is at
least one serious obstacle for the method which is the creation of
ripple structures due to the large difference in the thermal
expansion coefficients of nickel and graphite. The ripples have to
be delt with in order to obtain the planar graphene film topology
required for micro-patterning of electronic devices.
Epitaxial growth
Epitaxial growth of graphene is done by fabrication of a SiC
crystal from which the silicon atoms at the surface are
evaporated away by heating. The carbon atoms reconstruct to form
few-layer graphene. The layers are stacked rotationally over each
other, which preserves the Dirac properties of single-layer
graphene[7]. The process is done in ultrahigh vacuum (UHV) in
order to avoid oxidation. This method provides graphene suitable
for electronics but the requirement for UHV conditions makes it
expensive.
Colloidal suspensions
Production of graphenes from colloidal suspensions made from
graphite, derivatives of graphite and graphite intercalation
compounds is scalable and well-suited to chemical
functionalization. The methods using colloidal suspension afford
the possibility of low cost and high-volume production of
graphene, but of a lesser quality than the aforementioned methods.
One route to graphene sheets is via redox reactions [8]. First
graphite is oxidized in the presence of some strong acids and
oxidants. Then the graphite oxide is suspended in water and
intercalation of water molecules between graphene oxide sheets
occurs. Then a homogeneous colloidal suspension of graphene oxide
is achieved by simple sonication. Finally the aqueous graphene
oxide suspension is reduced and dried to produce an electrically
conductive black powder. However the oxygen can't be removed
completely and thus pristine graphene is not obtained.
Methods have been developed that avoid the oxygen impurities.
One uses intercalants that evaporate explosively when heated thus
creating thin graphite flakes that can be further separated with
proper intercalants and sonication [9]. The other method relies on
solvents for which the solvent-graphene interfacial interaction
energy matches that of graphene-graphene [10]. These both methods
produce graphenes of higher quality than other chemical routes but
they still fall short from yielding pristine graphene.
References
[1] Novoselov et al., Science 306, 666 (2004).
[2] Kim et al., Nature 457, 706 (2009).
[3] Berger et al., Science 312, 1191 (2006).
[4] Park & Ruoff, Nature Nanotech. 4, 217 (2009).
[5] Reina et al., Nano Lett. 9, 30 (2009).
[6] Cao et al., Preprint at
[7] Xuebin Li, Epitaxial graphene films on SiC: growth,
characterization, and devices (Georgia Institute of Technology,
2008).
[8] Stankovich et al., Carbon 45, 1558 (2007).
[9] Li et al., Nature Nanotech. 3, 538 (2008).
[10] Hernandez et al., Nature Nanotech. 3, 563 (2008).

Chirality identification in single walled carbon nanotubes
Jyri Petteri Rintala
Introduction
Carbon nanotubes (CNT), especially single walled (SWNT), can
be treated as a folded sheet of graphene. The way that the
graphene sheet is folded determines the properties of the tube and
the structure of the nanotube is described by an integer pair
(n,m), from which also the chiral vector and the diameter of
a SWNT can be derived. Because the synthesis of the CNTs always
produce a distribution of different chiralities instead of one
single chirality, it is important to be able to determine the
actual chirality of the tube in the sample. The methods used for
the determination of the chirality of CNTs are for example
resonant Raman spectroscopy, electron diffraction and high
resolution transmission electron microscopy (HRTEM).
Resonance Raman Spectroscopy
Raman spectroscopy has been widely used for characterizing
SWNTs because it is one of the most sensitive and informative
spectroscopic methods to analyze this kind of nanostructures.[1,2]
In particular, surface enhanced Raman spectra (SERS) of CNTs [3]
suggested that it could be possible to obtain a Raman spectrum
from an individual SWNT. In 2001 Jorio et al. presented an
observation of Raman spectrum from a single nanotube.[4] This
made it possible to study the dependence on diameter and chirality
of each feature of Raman spectra of SWNTs.
In order to analyze the resonance Raman spectra it is
necessary to compare the experimental data with prediction or to a
plot of the resonance transition energies as a function of tube
diameter for all SWNTs.[5] This so called Kataura plot shows that
the transition energies Eii of each band are approximately
inversely proportional to tube diameter dt.[6] In determining the
possible resonant tubes it is usually assumed that the SWNT should
have Eii within a resonant window of the energy of used laser El '
0.10 eV.[7-9] The diameter of the CNT is obtained from RBM
position by using equations presented in literature, for example
in reference [7].
Electron Diffraction
An electron diffraction pattern from a SWCNT has two sets of
principal reflections, appearing as two twisted hexagons. [10] One
originates from the top layer and the other from the bottom layer
as the electron beam passes through the tube. Due the periodicity
in axial direction, the scattering intensities on a set of lines.
Though the scattering intensities are observable on all layer
lines, the principal reflections of {1 0 0}* type bear the
strongest intensities. From these reflections rise to three layer
lines above and below the equatorial line, respectively.
[10] The scattering intensity distribution of the lines can
be calculated precisely. [11] The positions of the first two (or
three in some cases) peaks in the intensity distribution are used
to determine the orders of Bessel functions which directly give
the chiral indices of the measured tube. [10]
High Resolution Transmission Electron Microscopy
Conventional high-resolution transmission electron microscopy
(HRTEM) can produce detailed images of the local SWNT
structure.[12] This method has been extended to determine the
chirality of a SWNT by a combination of the real space phase
restoration and its Fourier transformation. [12,13] However, long
time focusing of electron beam on specimen would induce distortion
of SWNTs and cause unexpected experimental errors. [14] Fourier
transform operation is performed to the intact HRTEM image to
obtain its optical diffraction pattern and after this inverse
Fourier transform is performed to obtain atomic-resolved lattice
image by masking stigmatic diffraction lines related to the tube
chirality. Eventually through simulations and comparison to other
experimental data, the chiral angle and the diameter of CNT are
obtained. [14]
References
[1] Dresselhaus, M. S.; Eklund, P. C. Adv. Phys. 2000, 49,
705.
[2] Jorio, A.; Pimenta, M. A.; Souza Filho, A. G.; Saito, R.;
Dresselhaus, G.; Dresselhaus, M. S. New J. Phys. 2003, 5,
139.
[3] Kneipp, K.; Kneipp, H.; Corio, P.; Brown, S. D. M.; Shafer,
K.; Motz, J.; Perelman, L. T.; Hanlon, E. B.; Marucci, A.;
Dresselhaus, G.; Dresselhaus, M. S. Phys. Rev. Lett. 2000,
84, 3470
[4] Jorio, A.; Saito, R.; Hafner, J. H.; Lieber, C. M.; Hunter,
M.; McClure, T.; Dresselhaus, G.; Dresselhaus, M. S. Phys. Rev.
Lett. 2001, 86, 1118.
[5] Dresselhaus, M. S.; Dresselhaus, G.; Jorio, A. J. Phys. Chem.
C, 2007, 111, 17893.
[6] Kataura, H.; Kumazawa, Y.; Maniwa, Y.; Umezu, I.; Suzuki, S.;
Ohtsuka Y.; Achiba, Y. Synth. Met. 1999, 103, 2555.
[7] Araujo, P. T.; Doorn, S. K.; Kilina, S.; Tretiak, S.;
Einarsson, E.; Maruyama, S.; Chacham, H.; Pimenta, M. A.; Jorio,
A. Phys. Rev. Lett. 2007, 98, 067401.
[8] Maultzsch, J.; Reisch, S.; Hennrich, F.; Thomsen, C., Phys.
Rev. B 2005, 72, 205438.
[9] Jorio, A.; Saito, R.; Hafner, J. H.; Lieber, C. M.; Hunter,
M.; McClure, T.; Dresselhaus, G.; Dresselhaus, M. S. Phys. Rev.
Lett. 2001, 86, 1118.
[10] Liu, Z.; Qin, L.-C.; Chemical Physics Letters 2005,
408, 75'79
[11] Qin, L.-C. J. Mater. Res. 1994, 9, 2450.
[12] Meyer, R. R.; Friedrichs, S.; Kirkland, A. I.; Sloan, J.,
Hutchinson, J. L.; Green, M. L. H. J. of Microscopy 2003,
212 Pt 2, 152'157
[13] Friedrichs, S.; Sloan, J.; Green, M.L.H.; Hutchison, J.L.;
Meyer, R.R.; Kirkland, A. I. Phys. Rev. B 2001, 64, 045406.
[14] Zhu, H.; Suenaga, K.; Hashimoto, A.; Urita, K.; Iijima, S.
Chem. Phys. Lett. 2005, 412, 116-120

Toxicology of carbon nanostructures
Gianmario Scotti
Introduction
Fullerenes and especially carbon nanotubes (CNTs) are increasingly
leaving the exclusive domain of laboratories and into everyday
life. Applications such as displays, fuel cells and composite
materials (even cements) have varied levels of contact with human
body, and it is reasonable to expect that some of this
nanomaterial will be inhaled or ingested, or penetrate through
skin and other tissue. Given that industry and private entities
don't inherently have the best interest of humankind at their
heart, it is the duty of the scientific community to carefully
research and evaluate the effects that these new substances have
on the metabolism and life-functions, in the medium and long
terms.
Use of nanoparticles is, generally speaking, nothing new: gold and
other metal nanoparticles were used since ancient times for
staining glass, and metallic oxides and silica have been in use in
the cosmetic industry, in catalysts etc. for the past few decades
[1] [4]. From this to conclude that any nanoparticles made of such
oxides are innocuous, would be a mistake, as evidenced by the
adverse effects of asbestos [2]. Therefore shape/morphology and
size of nanostructures are relevant (also) for their
health-related action.
Research challenges
When researching the toxicity of carbon nanostructures, one is
facing a few difficulties: in vitro experiments don't necessarily
produce results relevant to the functioning of a complex organism
and can lead to both over- and underestimates. In vivo
experiments, on the other hand, are expensive, especially when
looking at long-term effects [1]. Still, in vitro experiments are
necessary to understand the mechanisms of toxicity of
nanomaterials [3]. Localization, in tissues and organs, of
absorbed carbon nanomaterials is difficult, while radiolabeling or
fluorescently tagging the nanostructures may alter their
properties [3]. One possible solution for radiolabeling was found
in 1994, by using 14C [9].
Another issue is finding proper control samples to test against
carbon nanostructures [3]. While carbon black has been used for
this purpose, its reactivity (e. g. with markers) may skew
measurements.
Light excitation of nanoparticles is of fundamental importance and
introduces another variable for the researcher to consider. The
excited nanostructures may react with substances present in the
ambient, causing the attachment of various functional groups; this
presents the need to test the toxicity and bio-action of disparate
classes of fullerene or CNT-based compounds. For instance,
C60(OH)24 causes increased growth of B
subtilis bacteria compared to non-hydroxilated C60
or conrol samples [1]. Furthermore, it is likely that excited
fullerenes or CNTs will behave differently in a living organism,
than those at the ground state, so the researcher should bear this
in mind in some cases. This further complicates the research
methodology.
It is not surprising that scientists are encountering such
challenges at this stage, as appreciable quantities of fullerenes
and CNTs have been produced only recently, and the amount of
studies is (as shown in the next section) undergoing exponential
growth in the last couple of years.
History of research
The few first published papers on carbon nanomaterials toxicity
appear in 1992-1993 [5] [6], and deal with C60
fullerenes. Tokuyama et al. [6] did a pioneering work not only
because they performed one of the first studies on toxicity of
carbon nanostructures, but also because they researched the
photo-activation of fullerenes with regards to bio-activity. The
article reports on the study of cytotoxicity of C60
carboxylic acid in vitro, on HeLa S3 cells. A marked cytotoxic
activity was observed when the sample was irradiated by 6 W
fluorescent "white" light, and none otherwise. Similarly, the
triethylamine salt of C60 carboxylic acid showed
DNA-cleaving activity only when photo-irradiated. The above
compounds were chosen as they are much more soluble in water than
C60 itself.
Since carbon nanotubes have been "officially discovered" in 1991,
research on their toxicology lags almost a decade. One of the
first articles to deal with CNT toxicity is by Huczko et al. [7];
the methodology involved is interesting, as the source material is
soot from a fullerene arc generator, containing both
C60 and CNTs. Both dermatological and pulmonary tests
on guinea pigs were performed, and the paper concludes that no
skin irritation or allergy was prodced after 72 hours, nor did the
soot with high-content of CNTs cause measurable inflammation in
the pulmonary system. In the name of historical accuracy it mast
be noted that the same authors have published, the year before
(1999), a short communication on their ongoing research [8].
As is the case in the early article by Tokuyama et al. [6],
research on carbon nanostructure toxicity often times goes
hand-in-hand with fullerene and CNT beneficial bio-activity or
medical use. In fact, all throughout the short history of the
research in the topic of toxicity, the research in benefits and
medical uses of carbon nanostructures went, more or less, at the
same speed, and a balanced and comparable if not equal, amount of
research data has been produced.
Doing a search for "toxicity study of carbon nanotubes" (without
quotes) on Elsevier, produces a remarkable result: about 90% of
the articles found in this way, have been published in or after
2006, and as of this essay's writing (mid-August 2009), the number
of articles published this year almost equals that of the previous
two years. This indicates that the interest in this topic is
enjoying an explosive growth.
More recent publications
In reference [10], it is reported that weakly water-soluble
C60 is much more cytotoxic to in vitro human cell
cultures, than C60(OH)24,
Na+2-3[C60O7-9(OH)12-15](2-3)-
or C3 (C60 derivatized with malonic
acid), all of which are highly water-soluble derivatives of
C60. The authors acknowledge the importance of light
activation of fullerenes, and opted to perform the experiments in
the dark. C60 was found to be toxic to human dermal
fibroblasts at a LC50 value of 20 ppb, which was 3-5
orders of magnitude less than for the derivatives. A very
interesting finding was that the level of toxicity is inverse to
the level of derivatization of the C60 cage. After
further experiments, the article concludes that the likely
mechanism of C60 cytotoxicity is peroxidation of the
cellular lipid bilayer, forming peroxy-radicals on its alkene
termini, causing its hydrophobicity and perforation. The ability
of C60 to provide oxygen radicals was also empirically
confirmed.
Monteiro-Riviere et al. [3] performed in vitro experiments on
human epidermal keratinocytes by exposing them to multi-walled
CNTs (MWCNT). A 0.4mg/ml dose after 48h caused 84% of the cells to
be penetrated by MWCNTs, indicating a high dermatological hazard
from these materials. Besides this, the article has found widely
divergent results when using carbon black controls from different
sources/vendors, underlying the need to find correct control
materials.
Smith et al. [11] introduced single-walled CNTs (SWCNT), with a
surfactant and sonication, into the water tanks with rainbow
trouts, and tested against freshwater and water with surfactant
only. They find that 0.1-0.5 mg/l SWCNTs cause marked increase in
ventilation rate, mucus secretion and enlarged mucocytes on the
gills, after 10 days of exposure.
In reference [12], a review is made of current (to October 2005)
studies in pulmonary toxicity of inhaled CNTs. It is suggested
that depostion and toxicity of CNTs (SW and MW CNTs, CNT "ropes"
and other aggregates) increases with the decrease of the
nanoparticles' aerodynamic diameter. Another conclusion is that
MWCNTs and SWCNTs can cause severe inflammatory and fibrotic
reactions.
Conclusion
The few articles presented in this essay all point to potential
dangers from exposure to carbon nanostructures. At the same time,
carbon nanostructures can be also very useful in medical
applications, so the research in this field is doubly rewarding.
This research is still young and finding solutions to
methodological challenges, but as the research effort expands (and
it is expanding exponentially), obstacles will be overcome and
more useful data generated.
References
[1] Challa S. S. R. Kumar: "Nanomaterials: Toxicity, Health and
Environmental Issues", Wiley-VCH 2007
[2] Roberta C. Barbalace. A Brief History of Asbestos Use and
Associated Health Risks. EnvironmentalChemistry.com. Oct. 2004.
Accessed on-line: 8/9/2009
http://EnvironmentalChemistry.com/yogi/environmental/asbestoshistory2004.html
[3] Nancy A. Monteiro-Riviere and Alfred O. Inman: "Challenges for
assessing carbon nanomaterial toxicity to the skin", Carbon
[0008-6223] yr:2006 vol:44 iss:6 pg:1070
[4] Maynard, A.D., Michelson, E. 2005. The nanotechnology consumer
products inventory. Woodrow Wilson International Center for
Scholars.
[5] Wagemann, K., Baselt, J.P.: Fullerenes - analysis and
evaluation A discussion paper prepared within the framework of the
analysis and evaluation of new innovative approaches to chemical
fundamental research (1992)
[6] Hidetoshi Tokuyama, Shigeru Yamago, Eiichi NakamuraTakashi
Shiraki and Yukio Sugiura: Photoinduced Biochemical Activity of
Fullerene Carboxylic Acid, J. Am. Chem. Soc. 1993, 115, 7918-7919
[7] Andrzej Huczko, Hubert Lange, Ewa Calko, Hanna
Grubek-Jaworska, Pawel Droszcz, Toshiaki Sogabe: On Some Aspects
of the Bioactivity of Fullerene Nanostructures, Electrochem. Soc.
Proceedings, Vol. 2000-11
[8] A. Huczko, H. Lange, E. Calko:,Short Communication:
"Fullerenes: Experimental Evidence for a Null Risk of Skin
Irritation and Allergy"; Fullerenes, Nanotubes and Carbon
Nanostructures, Volume 7, Issue 5
September 1999, pages 935 - 939
[9] Scrivens WA, Tour JM: Synthesis of 14C-labeled
C60, its suspension in water, and its uptake by human
keratinocytes; Journal of the American Chemical Society
[0002-7863] yr:1994 vol:116 iss:10 pg:4517
[10] Christie M. Sayes et al.: The Differential Cytotoxicity of
Water-Soluble Fullerenes; Nano Letters 2004, Vol. 4, No. 10 pp.
1881-1887
[11] Catherine J. Smith, Benjamin J. Shaw, Richard D. Handy:
Toxicity of single walled carbon nanotubes to rainbow trout,
(Oncorhynchus mykiss): Respiratory toxicity, organ pathologies,
and other physiological effects; Aquatic Toxicology 82 (2007)
94'109
[12] Julie Muller, Francois Huaux, Dominique Lison: Respiratory
toxicity of carbon nanotubes: How worried should we be?; Carbon 44
(2006) 1048'1056

The fullerene family and fullerene-based materials
Shulzhenka Yury
Fullerenes are a relatively new class of nanomaterials that
represent a third form of carbon (e.g. diamond and graphite) and
are spherical in shape like a soccer ball. The most common
fullerene sphere, called a "Buckyball," contains 60 carbon atoms
bound by single and double bonds that form a three-dimensional
geodesic spheroidal crystal. For these "empty cages", 60 or 70
carbon molecules are arranged in a cage structure and are water
insoluble unless derivatized with hydrophillic compounds. The
fullerene family has very appealing properties which can be
exploited alone or through the addition of atoms within the cage
or by the addition of surface chemistry. (1) There are many
identified possibilities for using these molecules in developing
new drugs or improving upon current drugs. For example, the
compounds can be used for specific targeting of cells and
locations within the body after intravenous or subcutaneous
injection. Likewise, certain fullerenes, such as the
Trimetasphere, contain metallic ions which help increase image
quality. And, since these metals are captured within the car-bon
cage, release into the body is not a pathway for these toxins
the cage carries the metal throughout elimination. Given the
plethora of capabilities and options that fullerenes and
Trimetaspheres bring to the field of nanomedicine, it is not
surprising the effect it is having on medical research and drug
delivery science. Chemists can now produce various derivatives of
fullerenes. For example, hydrogen atoms, halogen atoms, or organic
functional groups can be attached to fullerene molecules. Also,
metal ions, noble gas atoms, or small molecules can be trapped in
the cage-like structures of fullerene molecules, producing
complexes that are known as endohedral fullerenes. If one or more
carbon atoms in a fullerene molecule is replaced by metal atoms,
the resultant compound is called a fulleride. Some doped
fullerenes (doped with potassium or rubidium atoms, for example)
are superconductors at relatively high temperatures. The chemistry
of fullerenes, developed in these last years, has allowed
designing the properties of this family of molecules for specific
applications in materials science. One of the main tasks to build
up solid state devices based on fullerenes is the synthesis of
materials doped with a highly dispersed and homogeneous
distribution of fullerenes. Many of the peculiar photophysical
properties, such as the reverse saturable absorption used to
obtain a solid state optical limiter, are in fact lost in the
aggregates of fullerenes. Sol-gel processing allows preparing
inorganic oxides and hybrid organic-inorganic mate-rials at low
temperatures and presents an interesting alternative to organic
polymers to entrap molecules of the fullerene family in a solid
matrix. Porous inorganic solids and aerogels are also important
classes of materials that can be synthesized via sol-gel and can
act as hosts of fullerenes. (2) Discovery of fullerenes and their
soluble derivatives brought revolutionary break-throughs in the
fields of organic electronics and material science. Parent
fullerene C60 is the most advanced organic n-type semiconductor
that showed electron mobilities as high as 5-10 cm2 /V s when
measured in organic field effect transistors (OFETs). Organic
de-rivatives of fullerenes serve as solution processible n-type
semiconductors. I summarised the some literature data of the
fullerene-based materials. Electronic circuitry. Fullerene C60 and
its derivatives are widely used for design of OFETs and more
sophisticated electronic devices such as ring oscillators and
other functional integrated circuits. There are few companies on
the market that develop smart cards, smart tags and some other
consumable electronics built entirely from C60-based transistors.
Functionalized fullerenes are materials of choice for low cost
printable or-ganic electronics. Organic solar cells based on
conventional organic materials showed power con-version
efficiencies of less than ~1% in 2000. A remarkable breakthrough
in the field was achieved when fullerene-based materials were
applied. A rapid evolution of organic photovoltaics resulted in
power conversions efficiencies of >5% certified for single cells
by Konarka Technologies. Calculations revealed that power
conversion efficiencies of 10-14% are feasible for organic solar
cells based on appropriate photoactive materials. Organic
photodiodes and photodetectors. Photodiodes based on composites of
fullerene derivatives and conjugated polymers have advantages of
exceptionally high sensitivity, fast photoresponse, low or even
zero energy consumption and a low cost. Siemens AG demonstrated
position-sensitive photodetectors, two-dimensional image scanners
and digital cameras based on arrays of organic photodiodes. These
develop-ments are currently used in electronic equipment for
medicinal diagnostics, in particular, for X-ray imaging. Organic
light emitting diodes (OLEDs) comprising fullerene C60 as electron
transporting material will also be considered. Successful
application of the fullerene-based materials in organic
electronics resulted in appearance of a number of commercialized
products that prove unambiguously that fullerenes are not just
beautiful, they are useful". (3)
References
1. Fullerene Nanomedicines for Medical and Healthcare
Applications. Charles Gause. www.hhmglobal.com
2. Brusatin G., Innocenzi P. Journal of Sol-Gel Science and
Technology, Volume 22, Number 3, November 2001, pp. 189-204.
3. Fullerene-based materials for organic electronics. Razumov
V.F. www.ioffe.ru/IWFAC/2009/abstr/iwfac09. p010.pdf

Unique applications of mechanical toughness of nanotubes
Aleksi Sievänen
Introduction to material toughness
When talking about material toughness people are often not
sure what it actually means. Mathematically toughness of material
is the area underneath stress-strain curve Fig 1 [1]. On the other
words it is materials“s ability to absorb mechanical (or kinetic)
energy up to failure. Mathematical description is shown in Fig 2.
[2].

Figure 1
Figure 2
Material strength is closely related to material toughness. On
Fig. 1 ultimate tensile strength is the highest point where the
line is momentarily flat. So we can see that if we increase steel
strength we“ll end up lower total toughness values.
If we take a look to published theoretical and experimental
values of nanotubes mechanical properties (for example from
Wikipedia) [3] we can see that tensile strength of pure nanotubes
is 50-100 times higher than Stainless steel and elognation at
break is on almost same level.
When we add the fact that carbon nanotubes have one sixth of
Stainless steel density we start to understand how unique the
material mechanical properties of nanotubes theoretically are.
Combining strength and weight properties of nanotubes to
strength/weight ratio the multiplier will be amazing 300-600
related to high or medium carbon stainless steels.
Applications
How unique applications we can theoretically make out of such
supermaterial can be divided to two ways of thinking. We could use
the properties either to make existing constructions ultra light
or ultra tough. Ultra light products will help engineers in coming
energy consumption challenge e.g building Hydrogen cars,
ultraspeed flywheels. Ultra tough properties could lead to
constructions which have not been possible before e.g. Space
elevator [4]. But because the properties of nanotubes are so good
it might mostly be better to distribute the value to both
lightness and toughness. In practice it is not likely to be
possible to produce materials which consist only of carbon
nanotubes. This is the reason why the numbers shown on Table 1 for
nanotubes are too optimistic. It is more likely that nanotubes are
being used to enhance exisiting materials like concrete, ceramics,
plastics and composites. There is already variety of nano-enhanced
sport goods like golf shafts, golf balls, tennis rackets available
on marketplace and more will come for sure after manufacturing
technologies and engineers understanding maturize. On the other
hand totally new materials where nanotubes has been used will be
and are being developed. For decades material scientist have tried
to mimick spider silk to be able to create e.g. lightweight stab
or bullet proof fabrics.Thanks to nanotubes It seems that this
dream is now closer than ever. Nexia and US Army researchers has
reported fabrication of such fiber already 2002 [5]. Another
promising result has been reported in 2003 by Nanotech Institute
at university of Texas at Dallas [6].
History has showed that it will take still several years or
decades before new inventions, in this case the most unique
materials, are available in mass production and for normal
consumer. I personally hope to see some materials with high
wow-facor during my lifeteime. A stab-proof shirt like Frodo
weared in J.R.R. Tolkien“s Fellowship of the Rings would be nice.
References
[1] NTD resource center website retrieved August 10 2009 from:
http://www.ndt-ed.org/EducationResources/CommunityCollege/Materials/Mechanical/Toughness.htm
[2] Wikipedia.com, retrieved August 10 2009 from:
http://en.wikipedia.org/wiki/Toughness
[3] Wikipedia.com, retrieved August 10 2009 from:
http://en.wikipedia.org/wiki/Carbon_nanotube
[4] Liftport company website, retrieved August 10 2009 from:
http://www.liftport.com/
[5] Eureka Alert website retrieved
August 10 2009 from:
http://www.eurekalert.org/pub_releases/2002-01/nbi-nau011102.php
[6] Alan Dalton Nanostructured and molecular materials group
website, retrieved August 2009 from:
http://personal.ph.surrey.ac.uk/~phs1ad/Dalton_Home_Page/industrialscientist.pdf

Nanoweaving of nanotubes
Ilkka Sirjonen
Carbon nanotubes (CNTs) have been known for some time now.
Scientist are constantly conjuring up new ideas on how to use
them. These range from printable batteries to memory elements in
electronics and mixing them in epoxy to form very light composite
materials. Because of the great mechanical and electromechanical
properties of CNTs they are a very interesting material not only
in nanoscale structures but also in macroscale products. One of
the ideas involves spinning them into a yarn that can be used in
various textiles. The nanotube yarn, in contrast to common carbon
fibers, can be bent and even tied into knots without breaking.
Therefore it could be used to make fiber supercapacitors [1] or
very durable clothing, possibly even bullet-proof vests.
The process of assembling bundles of single-walled carbon
nanotubes (SWNTs) into a continuous fiber is actually quite simple
[2]. SWNTs produced with the electric-arc technique are dispersed
and sonicated in a solution of sodium dodecyl sulfate (SDS), where
in optimized concentrations a homogenous dispersion is obtained.
Then this solution is injected through a capillary into a stream
of a solution containing polyvinylalcohol (PVA). The flow of the
PVA solution orients the SWNT-bundles in the direction of the flow
and the bundles stick together because the PVA solution does not
stabilize against attracting forces between the tubes and bundles
like SDS solution does. The result is a continuous ribbon which
remains stable. The ribbons can even be washed with pure water to
desorb SDS and PVA. The setup used to make nanotube ribbons is
shown in Fig. 1.
![]()
Nanotube yarn can also be dry spun from multiwalled carbon
nanotubes (MWNTs) fabricated using chemical vapor deposition (CVD)
-technique [3]. In this method nanotubes are drawn from a MWNT
"forest" on a catalyst coated substrate (Fig. 2). The drawn
nanotubes are twisted into a yarn very similarly to spinning yarn
out of wool or cotton. Hence the geometry of the yarn is similar
to that of wool. While typical wool or cotton yarn cross section
contains up to about 100 fibers the nanotube yarn contains aout
100,000 MWNTs.
![]()
The nanotube yarns are highly flexible and can be tightly
knitted and knotted which is a major advantage compared to most
polymer fibers, where knotting degrades the strength of the fiber
severely [3]. The yarns can also be easily plied like ordinary
ropes to make them thicker. SEM images of single-, two ply-,
four-ply-, and knitted and knotted MWNT yarns are shown in Fig. 3.
The yarns retain their flexibility even when exposed to
temperatures of 450°C for long periods or when immersed in liquid
nitrogen.
![]()
Other properties of nanotube yarns are quite impressive. They
can have tensile strengths on the order of 1.8 GPa which matches
that of spider silk [1]. The supercapacitors made from coated
nanotube fibres can achieve capacitance and energy-storage density
of commercial supercapacitors.
The strength, toughness, conductivity, mechanical energy
damping capability, flexibility and small diameter (~2% of that of
human hair) makes the nanotube yarns a very interesting material
for a variety of uses. They could replace metal wires in in
electronic textiles and eliminate the rigidity of metal wire
-containing textiles. Electronic applications could include
distributed sensors, electronic interconnects, electromagnetic
shields, antennas and batteries.
References
[1] Super-tough carbon-nanotube fibers, Alan B. Dalton et al.,
Nature 423 (2003).
[2] Macroscopic Fibers and Ribbons of Oriented Carbon Nanotubes,
Brigitte Vigolo, et al., Science 290 (2000).
[3] Multifunctional Carbon Nanotube Yarns by Downsizing an Ancient
Technology, Mei Zhang et al., Science 306 (2004).

Application of nanoparticles in plant biotechnology
Anna Timchenko
Nanotechnology is a collective term for a wide range of
relatively novel technologies; the main unifying theme is that it
is concerned with matter on the nanometre scale (Greek nanos
means dwarf). Bionanotechnology or nanobiotechnology is a
sub-section of nanotechnology. Bionanotechnology involves the
exploitation of biomaterials, devices or methodologies on the
nanoscale [1]. In recent years, nanoparticles with sizes typically
below 100 nm, have been applied in several fields of bioscience
and biomedicine, with an increasing number of commercial
applications. Advances have been made in the field of biomedicine,
including the development of tools for pathogen bio-detection,
tissue engineering and MRI contrast enhancement. Special interest
have been focused on those applications developed for targeted
delivery of substances and drugs, implying direct movement of
nanoparticles to specific organs.
The possibility of targeting the movement of nanoparticles to
specific sites of an organism paves the way for the use of
nanobiotechnology in the treatment of plant diseases that affect
specific parts of a plant. Different procedures have made use of
nanoparticles in plants, such as the controlled release of
bioactive substances in solid wood and plant transformation
through bombardment with gold or tungsten particles coated with
plasmidic DNA[2].
Two areas of nanoparticles application in plant
biotechnology.
One of the most well-known areas of research in plant
biotechnology is the application of nanomaterials in the
agricultural and food industry, where nanoparticals can be used as
new tools for the molecular treatment of diseases, rapid disease
detection, enhancing the ability of plants to absorb nutrients
etc. Smart sensors and smart delivery systems can help the
agricultural industry combat viruses and other crop pathogens. For
example, devices could be used to identify plant health issues
before these become visible to the farmer. Such devices may be
capable of responding to different situations by taking
appropriate remedial action. If not, they will alert the farmer to
the problem. In this way, smart devices will act as both a
preventive and an early warning system. Such devices could be used
to deliver chemicals in a controlled and targeted manner in the
same way as nanomedicine has implications for drug delivery in
humans [3].
The chemical noise arising in ecosystems when using the most
of chemicals of plants' protection from pests and pathogens result
in unstable state of biocenoses. And this fact in its turn affects
adversely steady position of gene pool. Besides, series of
chemicals (herbicides, fungicides, germicides, insecticides,
synthetic growth-regulating and plant development chemicals)
polluting soil, atmosphere and water bodies affect negatively
health of people and development of such important branches of
productions as plant cultivation, cattle breeding etc [4].
Technologies such as encapsulation and controlled release methods,
have revolutionised the use of pesticides and herbicides. Many
companies make formulations which contain nanoparticles within the
100-250 nm size range that are able to dissolve in water more
effectively than existing ones (thus increasing their activity).
Other companies employ suspensions of nanoscale particles
(nanoemulsions), which can be either water or oil-based and
contain uniform suspensions of pesticidal or herbicidal
nanoparticles in the range of 200-400 nm. These can be easily
incorporated in various media such as gels, creams, liquids etc,
and have multiple applications for preventative measures,
treatment or preservation of the harvested product. One of the
worlds largest agrochemical corporations, Syngenta, is using
nanoemulsions in its pesticide products [3].
In my area of research the most interesting application of
nano biotechnology is the process of delivering genes into plant
tissue. This process involves two stages. First the gene is
inserted into plant cell tissue, and then afterwards chemicals are
introduced into the plant to trigger the genes function. The
two-stage process has been imprecise and the presence of chemicals
can be harmful to the plant. Torney et al. made the solution of
this problem, using mesoporous Nanoparticles. These Nanoparticles
both introduce the gene and activate it at the same time, in a
precise and controlled manner and without toxic after effects.
Scientists could potentially use this as an aid in imaging
analysis of plants which have been activated with the appropriate
materials.
Earlier Lins research group [5] developed technology with the
use of porous, silica nanoparticle system. Spherical in shape,
the particles have arrays of independent porous channels. The
channels form a honeycomb-like structure that can be filled with
chemicals or molecules.
In previous studies, this group successfully demonstrated that
the caps can be chemically activated to pop open and release the
cargo inside of animal cells. This unique feature provides total
control for timing the delivery The team's first attempt to use
the porous silica nanoparticle to deliver DNA through the rigid
wall of the plant cell was unsuccessful. The technology had worked
more readily in animals cells because they don't have walls. The
nanoparticles can enter animal cells through a process called
endocytosis - the cell swallows or engulfs a molecule that is
outside of it. The biologists attempted to mimic that process by
removing the wall of the plant cell (called making protoplasts),
forcing it to behave like an animal cell and swallow the
nanoparticle. It didn't work. They decided instead to modify the
surface of the particle with a chemical coating. The coating
induces the plants to swallow the particles, effectively ingesting
them inside the plant cell walls where the genes could be
inserted. Most plant transformation, however, occurs with the use
of a gene gun, not through endocytosis. In order to use the gene
gun to introduce the nanoparticles to walled plant cells, the
chemists made another clever modification on the particle surface.
They synthesized even smaller gold particles to cap the
nanoparticles. These "golden gates" not only prevented chemical
leakage, but also added weight to the nanoparticles, enabling
their delivery into the plant cell with the standard gene gun. The
biologists successfully used the technology to introduce DNA and
chemicals to Arabidopsis, tobacco and corn plants.
Thus, being able to penetrate the cell wall of the plant
enables biologists to view the world of plant biology in all of
its complex and intricate detail, opening vast new frontiers of
discoveries for agriculture and other industries that rely on
biotechnology.
References
[1] Nicole F. Steinmetz and David J. Evans, Utilisation of plant
viruses in bionanotechnology, Organic & Biomolecular Chemistry
(2007).
[2] Eduardo Corredor, Pilar S Testillano, Marķa-José Coronado,
Nanoparticle penetration and transport in living pumpkin plants:
in situ subcellular identification, BMC Plant Biology (2009).
[3] Francois Torney, Brian G. Trewyn, Victor S.-Y. Lin, and Kan
Wang, Mesoporous Silica Nanoparticles Deliver DNA and Chemicals
into Plants, Nature Nanotechnology (2007).
[4] Muzaffar Sharipov, Bio-nanotechnology in preparation of rice
seeds and increase of tolerance of rice crop to diseases and
unfavorable environmental factors (unpublished).
[5] Supratim Giri, Brian G. Trewyn, Victor S.-Y. Lin, Mesoporous
Silica Nanomaterial-based Biotechnological and Biomedical Delivery
Systems, Nanomedicine (2007).

Application of carbon nanotubes in biomedicine
Maria Timchenko and Liliia Fakhranurova
Carbon nanotubes are a new class of nanomaterials that have
immense potential in the field of biomedicine. The application of
CNT in the field of carrier-mediated delivery has become possible
after the recent discovery of their capacity to penetrate into the
cells. Besides their versatile physicochemical features enable the
covalent and noncovalent introduction of several pharmaceutically
relevant entities and allow for rational design of novel candidate
nanoscale constructs for drug development. CNTs can be
functionalized with different functional groups to carry
simultaneously several moieties for targeting, imaging, and
therapy [1-3]. Functionalized carbon nanotubes (f-CNT) have been
demonstrated to deliver proteins, nucleic acids, drugs, antibodies
and other therapeutics. So besides the of CNT applications as
biosensors, composite materials, molecular electronics and so on,
one use of CNTs is as new carrier systems for the delivery of
therapeutic molecules and tissue engineering.
This essay focuses on applications of carbon nanotubes in drug
delivery and tissue engineering
1. Nanotubes as nanocarrier-based delivery
systems
Drug delivery is a rapidly growing area that is now taking
advantage of nanotube technology. This is due to the fact that
many potential low molecular weight drugs have bad
bioavailability, pure solubility, small half-life and intrinsic
toxicity [4]. Systems being used currently for drug delivery
include dendrimers, polymers, and liposomes, but carbon nanotubes
present the opportunity to work with effective structures that
have high drug loading capacities and good cell penetration
qualities. These nanotubes function with a larger inner volume to
be used as the drug container, large aspect ratios for numerous
functionalization attachments, and the ability to be readily taken
up by the cell [1]. Because of their tube structure, carbon
nanotubes can be made with or without end caps, meaning that
without end caps the inside where the drug is held would be more
accessible. The drug encapsulation has been shown to enhance water
dispersibility, better bioavailability, and reduced toxicity.
Encapsulation of molecules also provides a material storage
application as well as protection and controlled release of loaded
molecules [5]. All of these result in a good drug delivery basis
where further research and understanding could improve upon
numerous other advancements, like increased water solubility,
decreased toxicity, sustained half-life, increased cell
penetration and uptake, all of which are currently novel but
undeveloped ideas. It should be noticed that the possibility of
developing CNT for biomedical applications became a reality after
a series of powerful methodologies for their functionalization
were described [6]. Different approaches have been proposed to
render the nanotubes soluble and compatible with physiological
conditions. This is a fundamental issue for their integration into
living system environments. A critical parameter to determine
biocompatibility is the degree of toxicity of all CNT materials.
This is a key issue which is currently under careful and extensive
examination from various laboratories. Observations reported by
many groups have shown that functionalization remarkably reduces
the cytotoxic effects of CNT while increasing their
biocompatibility [1]. The evidence gathered so far highlights that
the higher the degree of CNT functionalization, the safer is the
material, particularly compared to pristine, purified CNT, thus
offering the potential exploitation of nanotubes for drug
administration. The CNT functionalized covalently with small
molecular weight molecules seem to penetrate plasma membranes to a
considerable extent via an energy-independent mechanism and cross
the membrane in a passive way acting like tiny needles. For
example, carbon nanotubes could be functionalized with
antibiotics. Amphotericin B is an antimycotic agent used against
particularly resistant fungal strains [1]. It is, however, of
limited use because it is highly toxic to mammalian cells, likely
due to its low solubility in water and its tendency to aggregate
and form pores in the cell membrane. The conjugation of
amphotericin B to carbon nanotubes could modulate its properties
in terms of toxicity and antimycotic efficiency. Another
application of CNT is the anti-cancer therapy. For instance, f-CNT
could contains a methotrexate molecule together with a fluorescein
probe. Methotrexate is a well-known and potent anticancer agent,
used also to cure autoimmune diseases [1] However, methotrexate
suffers of low bioavailability and toxic side effects. Therefore,
an increased bioavailability and a targeted delivery are highly
desirable. f-CNT might offer the possibility for improving
bioavailability and, in the presence of a targeting unit, to
address specifically cancer cells. Besides, single-walled CNT were
functionalized with a substituted carborane cage for boron neutron
capture therapy. The biodistribution study on different tissues
showed that water-soluble carborane nanotubes were concentrated
more in tumor cells than in other organs when administered
intravenously. These results were preliminary although also
promising for future applications of carbon nanotube boron-based
agents for effective treatment of cancer [1]. Another class of
carbon nanotube-based therapeutic candidates consists of their
constructs with synthetic peptide for immune system activation.
The decoration of functionalized nanotubes with B and T cell
peptide epitopes can generate a multivalent system able to induce
a strong immune response [1]. Peptides can be connected to the
tubes using the chemoselective approach.
2. Applications of carbon nanofibers in tissue
engineering
In the past decade, nanomaterials have been developed and explored
as potential scaffolds for tissue engineering. By virtue of their
high surface area and porosity, they have the potential to provide
enhanced cell adhesion and by virtue of the similarity of their 3D
architecture to natural extracellular matrix, they provide an
excellent micro/nano environment for cells to grow and perform
their regular functions [7]. Many nanomaterials (natural and
synthetic) have been used for bone, cartilage, ligament, and
skeletal muscle tissue engineering. Carbon nanofibers have
exceptional mechanical properties (three times that of bone
tissue), thereby giving a strong rationale to investigate them for
application in orthopedic or dental tissue engineering. Further,
carbon nanofibers have also been shown to exhibit excellent
conductivity, which might make them potential candidates for
neural tissue engineering applications. The carbon-nanofiber-based
implants can surpass in some ways the conventional metal alloy
implants used in orthopedics, as they have excellent
cytocompatibility properties and complications associated with
leachables in the form of metal ions released from implants do not
arise. In terms of mechanical properties, carbon nanofibers
possess a Youngs modulus of 2 TPa, which is significantly higher
than that of bone, whereas the tensile strength of carbon
nanofibers almost equals that of bone. Therefore, Price et al
explored the possibility of using carbon nanofibers for bone
tissue engineering. They compared osteoblast adhesion on carbon
nanofibers with that of conventional carbon fibers and showed
greater osteoblast adhesion on carbon nanofibers. To determine the
properties that caused enhanced adhesion on carbon nanofibers, the
authors studied osteoblast adhesion on carbon nanofibers. Carbon
nanofibers also showed enhanced osteoblast adhesion compared with
conventional carbon fibers. Due to their electrical conductivity,
carbon nanofibers were initially explored as electrically
conducting fibers, in nanoelectronic devices, field emitters, and
also in reinforcement. More recently, due to their conductivity,
carbon nanofibers are being explored as potential candidates for
neural tissue engineering. To determine the cytocompatibility of
carbon nanofibers as neural implants, Therefore, carbon nanofibers
need to be investigated further to establish their potential use
in neural tissue engineering.
Future steps
Carbon nanotubes are still a relatively unexplored area, and the
rapidly advancing fields that they are involved in can still be
pushed farther. Nanotubes are extremely versatile since they can
be included in numerous different fields because of their great
material properties. Any amount of improvements can be made to
carbon nanotubes through various techniques. For example, it was
shown that by electrospinning and plasma-functionalizing
single-walled nanotubes, adhesion to surrounding polymer matrices
was greatly improved along with the tensile properties of the
nanotubes. Also, we know that most nanotubes are cleared from the
body very quickly after being distributed throughout. This
decreases the chances of higher toxicity levels in the blood. Many
other properties increase the number of options available with
carbon nanotubes. The good functionalization of carbon nanotubes
allows us to attach a number of groups to the tubes for different
systems. Radioactive labels could be attached for use in
bioimaging. As mentioned before, fluorescence is already observed
in normal carbon nanotubes, but attaching labels allows for a
greater imaging window. This labeling capability can also be used
for targeting purposes. For example, attaching targeting groups to
the carbon nanotubes could open up doors for very specific drug
delivery systems. It was shown that carbon nanotubes were used to
deliver drugs to specific cancer cells of the epithelium, and this
was accomplished efficiently. This targeted killing of cancer
cells shows promise for numerous other improvements in cancer
therapy and treatment as well as the treatment of various
infectious diseases. With targeted nanotubes used for drug
delivery, specific cells could be aimed at to take up the carbon
nanotubes, as was shown with brain tumor cells. Also with these
studies, minimal toxicity was found when multi-walled carbon
nanotubes were injected into mice. Carbon nanotubes seem to be a
valuable option when considering such applications as drug
delivery or bio-imaging because they are readily functionalized,
display excellent material properties, can be used as imaging
agents or sensors, and keep the door open for many future
advances.
Conclusion
Although applications of nanotechnologies in biomedicine are
only in the early stages of development, the possibilities offered
by using these nanomaterials for treatment and diagnosis of CNS
disorders are outstanding. Various nanomaterials and nanodevices
can be used in neural regeneration, neuroprotection, and targeted
delivery of drugs and macromolecules across the bloodbrain
barrier, in tissue engineering and many other. These systems have
significant potential for clinical applications. All together,
these works lay an important foundation for future studies into
improving diagnosis and therapy of human disease.
References
[1] M. Prato et al., Acc.Chem.Res. 41, 60 (2008).
[2] R.P. Feazell et al., J.Am.Chem.Soc. 129, 8438 (2007).
[3] S.S. Suri et al., J.Occupational Medicine and Toxicology 2, 16
(2007).
[4] J.E. Kipp Int. J. Pharm. 284 109 (2004).
[5] A. Kulamarva Nanotechnology. 20, 25612 (2009).
[6] D. Tasis Chem. Rev. 106, 1105 (2006).
[7] R. Vasita Inter.J. of Nanomedicine. 1, 15 (2006).

Raman spectroscopy of graphene
Matti Tomi
Introduction
Since the experimental discovery of graphene in 2004 [1],
Raman spectroscopy has played an important role in the research of
few-layer graphene. Scientists familiar with carbon nanotubes knew
that Raman analysis was a powerful tool in chirality
determination, which probably led to the rapid adoption of the
Raman technique for graphene samples. Initially, Raman
spectroscopy was mostly used to determine the number of layers in
a graphene sheet, as it's a non-invasive and high-throughput
method for sample characterization. Later on, more versatile and
insightful experiments have been constructed to better utilize the
vast potential of Raman measurements.
Raman spectroscopy
Raman spectroscopy is based on the study of inelastic
scattering of photons when they interact with elementary
excitations of a target material. Such an excitation is typically
a phonon, but it can also be a polariton, a plasmon, a coupled
plasmon–phonon mode, or a single electron or hole
excitation. The amount of energy transferred between the incident
photon and the sample corresponds to an energy shift—and
thus a frequency shift—in the scattered photon. A plot of
the intensity of the scattered light as a function of this energy
shift is called a Raman spectrum and can provide information on
the lattice dynamics and electronic properties of the sample. This
Raman effect was predicted by Adolf Smekal in 1923 but was first
observed by Sir Chandrasekhara Raman in 1928, using a filtered
beam of sunlight as the photon source and his eye as the detector.
Modern setups typically use one or more semiconductor lasers
operating in the visible, near-infrared, or near-ultraviolet
regime and a charge-coupled device (CCD) for multichannel
detection. [2]
Raman spectrum of graphene
The Raman spectrum of high-quality graphene (and graphite) has
two characteristic peaks that stand out (Fig. 1), called the
G-peak and the 2D-peak (some earlier sources refer to the 2D-peak
as the G'-peak). The G-peak corresponds to an optical phonon in
the center of the Brillouin zone (Γ) with an energy shift of
~1580 1/cm which is slightly dependent on sheet thickness,
chemical and electrostatic doping, and temperature. The origin of
the 2D-peak is a bit more complicated, as it involves the creation
of an electron–hole-pair near the K point. The excited
electron (or the hole) is then scattered twice by zone-boundary
phonons with equal energies (~1335 1/cm) but opposite momenta.
When the electron–hole-pair recombines, a photon is emitted
with an energy that is shifted ~2670 1/cm relative to the incident
photon, giving rise to the 2D-peak. Because the wave vector is
conserved in every step of the process and the wave vector of
light (~105 1/cm) is much smaller than that of the
Brillouin zone boundary (~108 1/cm for graphene), it
follows that no first-order D-peak will emerge from a perfect
infinite lattice. However, near the edges of a sheet and in
defective graphene, one of the electron (or hole) scattering
events can be elastic, resulting in a visible D-peak at ~1335
1/cm. As the D- and 2D-peaks are directly related to the band
structure of graphene near the K points, their energy shifts and
line shapes depend on the incident photon energy and on the
electronic band details. [3,4]
 Figure 1:
Raman spectra of graphite and single-layer graphene. Dashed line
shows the spectrum at the edge of the monolayer with a clear D
peak present. The graphite spectrum has been downscaled to achieve
similar G peak height. Figure 1:
Raman spectra of graphite and single-layer graphene. Dashed line
shows the spectrum at the edge of the monolayer with a clear D
peak present. The graphite spectrum has been downscaled to achieve
similar G peak height.
Information in the spectra
The evolution of graphene's band structure with an increasing
number of layers enables one to determine the thickness of a
few-layer graphene sheet based on the 2D-peak line shape (Fig. 2).
Monolayer graphene has one valence band and one conduction band,
and due to their quasi-linear nature near K the possible electron
and hole scattering resonances have almost exactly the same
energy. Therefore, monolayer graphene has a rather narrow
single-component 2D-peak. When the number of layers increases, the
electronic bands split, enabling several transitions with slightly
different energies. Bilayer and trilayer 2D-peaks can be fitted
with 4 and 6 Lorentzian components, respectively, and eventually
in bulk graphite the infinitely many components form a continuum
band that seemingly consists of two wide Lorentzians [4]. In
addition to thickness determination, Raman spectroscopy can be
used to extract information on lattice defects and impurities on
the sheet, utilizing the existence and intensity of the D-peak.
With micro-Raman equipment this type of analysis can be refined to
include spatial resolution, allowing the examination of a sheet's
homogeneity. The shift of the G-peak has been used to provide
information on chemical and electrostatic doping of graphene
devices [5,6], as well as to determine that the thermal
conductivity of a suspended monolayer is astonishingly high, ~5000
W/mK [7]. Raman spectroscopy also enables the study of stacking
order in multilayer graphene samples [8], electron and phonon
dispersion measurements by varying the incident photon energy [9],
and possibly even chirality determination of graphene nanoribbons
[10]. All in all, Raman spectroscopy is probably the single most
versatile tool for probing graphene at the moment, with no decline
visible on the horizon.
 Figure 2:
Comparison of Raman 2D-peaks of monolayer, bilayer, trilayer, and
bulk graphite. The substrate (Si with 250 nm SiO2) and
laser wavelength (532 nm) are the same for all samples. Figure 2:
Comparison of Raman 2D-peaks of monolayer, bilayer, trilayer, and
bulk graphite. The substrate (Si with 250 nm SiO2) and
laser wavelength (532 nm) are the same for all samples.
References
[1] K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y.
Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, "Electric
Field Effect in Atomically Thin Carbon Films", Science 306,
666–669 (2004).
[2] G. Bauer and W. Richter (Eds.), Optical Characterization of
Epitaxial Semiconductor Layers (Springer-Verlag, Berlin
Heidelberg, 1996).
[3] A. C. Ferrari, "Raman spectroscopy of graphene and graphite:
Disorder, electron–phonon coupling, doping and nonadiabatic
effects", Solid State Communications 143, 47 (2007).
[4] L. M. Malard, M. A. Pimenta, G. Dresselhaus and M. S.
Dresselhaus, "Raman spectroscopy in graphene", Physical Reports
473, 51 (2009).
[5] C. Casiraghi, S. Pisana, K. S. Novoselov, A. K. Geim and A. C.
Ferrari, "Raman fingerprint of charged impurities in graphene",
Applied Physics Letters 91, 233108 (2007).
[6] J. Yan, Y. Zhang, P. Kim and A. Pinczuk, "Electric Field
Effect Tuning of Electron-Phonon Coupling in Graphene", Physical
Review Letters 98, 166802 (2007).
[7] A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan,
F. Miao and C. N. Lau, "Superior Thermal Conductivity of
Single-Layer Graphene", Nano Letters 8(3), 902 (2008).
[8] L. G. Cançado, K. Takai, T. Enoki, M. Endo, Y. A. Kim,
H. Mizusaki, N. L. Speziali, A. Jorio and M. A. Pimenta,
"Measuring the degree of stacking order in graphite by Raman
spectroscopy", Carbon 46, 272-275 (2008).
[9] D. L. Mafra, G. Samsonidze, L. M. Malard, D. C. Elias, J. C.
Brant, F. Plentz, E. S. Alves and M. A. Pimenta, "Determination of
LA and TO phonon dispersion relations of graphene near the Dirac
point by double resonance Raman scattering", Physical Review B 76,
233407 (2007).
[10] J. Zhou and J. Dong, "Vibrational property and Raman spectrum
of carbon nanoribbon", Applied Physics Letters 91, 173108 (2007).

Effect of interlayer coupling and edges on the electronic
structure of graphene and graphite
Andrii Torgovkin
Graphene is a flat layer of carbon atoms arranged in a
hexagonal lattice with two carbon atoms per unit cell. It is a
monolayer of graphite. Understanding the electronic structure of
graphene is the starting point for finding the band structure of
graphite. Of the four valence states, three sp2 orbitals form a
 state with three neighboring carbon atoms, and one p orbital
develops into delocalized
state with three neighboring carbon atoms, and one p orbital
develops into delocalized  and and
 states that form the highest occupied valence band and the lowest
unoccupied conduction band. The
states that form the highest occupied valence band and the lowest
unoccupied conduction band. The  and and
 states of graphene are degenerate at the corner (K point) of the
hexagonal Brillouin zone (BZ) (pic.1). Undoped graphene is a
semimetal (or zero-gap semiconductor), because although there is a
state crossing at Fermi energy, the density of states there is
zero and conduction is possible only with thermally excited
electrons at finite temperature. Moreover, the particular band
structure at the BZ boundary (that is, a linear dispersion) leads
to zero effective mass at the point where the valence and
conduction band meet together (six points) [1]. Due to this linear
dispersion relation at low energies, electrons and holes near
these six points, two of which are inequivalent, behave like
relativistic particles described by the Dirac equation for spin
1/2 particles (Dirac fermions) [2].
states of graphene are degenerate at the corner (K point) of the
hexagonal Brillouin zone (BZ) (pic.1). Undoped graphene is a
semimetal (or zero-gap semiconductor), because although there is a
state crossing at Fermi energy, the density of states there is
zero and conduction is possible only with thermally excited
electrons at finite temperature. Moreover, the particular band
structure at the BZ boundary (that is, a linear dispersion) leads
to zero effective mass at the point where the valence and
conduction band meet together (six points) [1]. Due to this linear
dispersion relation at low energies, electrons and holes near
these six points, two of which are inequivalent, behave like
relativistic particles described by the Dirac equation for spin
1/2 particles (Dirac fermions) [2].

Pic.1 Calculated band structure of graphene [3]
In the structure of graphite, graphene hexagons are stacked in
AAA, ABA, and ABC stacking modes. The strong in-plane C-C bonds
and weak interlayer interactions are present. The origin of the
strong intralayer interaction is the combination of sp2  and and
 bonds. The weak interlayer interactions are due to the overlap of
bonds. The weak interlayer interactions are due to the overlap of
 bonds between adjacent graphene layers (see a bonding structure of
a single graphene layer on picture 2) [4]. The interlayer orbital
interactions are responsible for the conductivity in graphite,
since without them there are would be zero-density of states at
Fermi level. So to find out the band structure of graphite the
possible model (here tight-binging plus dispersion model is
considered) should take into account tight-binging description of
intra- and interlayer interactions with description of interlayer
dispersion.
bonds between adjacent graphene layers (see a bonding structure of
a single graphene layer on picture 2) [4]. The interlayer orbital
interactions are responsible for the conductivity in graphite,
since without them there are would be zero-density of states at
Fermi level. So to find out the band structure of graphite the
possible model (here tight-binging plus dispersion model is
considered) should take into account tight-binging description of
intra- and interlayer interactions with description of interlayer
dispersion.

Pic. 2.Bonding structure of a graphene layer [4]
If the forms of graphite AAA, ABA and ABC are compared from
the tight-binging dispersion model (pic. 3), one can conclude,
that in ABA graphite the tight-binding contribution to the
calculated interlayer energy is anisotropic in ABA stacking over
AAA, whereas the dispersion contribution is more isotropic and
does not discriminate between the stacking forms.

Pic.3 Tight-binding plus dispersion (TBD) interplanar energy
curves for AAA and ABA graphite, shown along with the separate
tight-binding (TB) and dispersion (D) contributions to the
interplanar energy of each stacking form.
The result of the modeling is shown on pic.4. Here the
dispersion interaction potential was counted in a form of the
Tang-Toennies function:


Pic4. Occupied band structure of ABA graphite.
One can see that the splitting of  -band of
ABA graphite occurs in reciprocal space. There is no such
splitting for AAA graphite [5]. -band of
ABA graphite occurs in reciprocal space. There is no such
splitting for AAA graphite [5].
References
[1] T. Ohta, A. Bostwick, T. Seyller, K. Horn, E. Rotenberg.
Controlling the Electronic Structure of Bilayer Graphene. Science
313, 951 (2006).
[2] G.W. Semenoff Condensed-Matter Simulation of a
Three-Dimensional Anomaly. Physical Review Letters 53: (1984)
[3] G.W. Hanson. Fundamentals of nanoelectronic. (2008)
[4]W.A. Goddard. Handbook of nanoscience, engineering and
technology. (2002)
[5]A. H. R. Palser. Interlayer interactions in graphite and carbon
nanotubes. PCCP (1999)

Identifying and modifying the edge structure of graphene
Olga Trubienko
Graphene is a one-atom-thick planar sheet of sp2-bonded carbon
atoms that are densely packed in a honeycomb crystal lattice. It
can be viewed as an atomic-scale chicken wire made of carbon atoms
and their bonds. The name comes from GRAPHITE + -ENE; graphite
itself consists of many graphene sheets stacked together.
The carbon-carbon bond length in graphene is approximately
0.142 nm. Graphene is the basic structural element of some carbon
allotropes including graphite, carbon nanotubes and fullerenes. It
can also be considered as an infinitely large aromatic molecule,
the limiting case of the family of flat polycyclic aromatic
hydrocarbons called graphenes.
Measurements have shown that graphene has a breaking strength
200 times greater than steel, making it the strongest material
ever tested
Graphene has attracted great attention not only because it is
the ideal material to study the fundamental properties of 2D
nanostructures, but also for its potential applications in future
electronic devices. The exceptionally high crystallization and
unique electronic properties make graphene a promising candidate
for ultrahigh speed nanoelectronics
Graphene nanoribbons (GNR) have been receiving remarkable
attention. It was predicted that GNR with certain edge chirality
would open the bandgap and show distinguish magnetic, optical and
superconductive properties. The bandgap opening of GNR has already
been experimentally verified.
All these peculiar properties are strongly dependent on the
edge chirality (zigzag or armchair). Current technique to
fabricate such well defined GNR is e-beam lithography from
graphene sheet, in which the determination of the graphene crystal
orientation and edge chirality is highly desired. There are
several conventional methods: TEM, XRD, STM and other.
Transmission electron microscopy (TEM) is a microscopy
technique whereby a beam of electrons is transmitted through an
ultra thin specimen, interacting with the specimen as it passes
through. An image is formed from the interaction of the electrons
transmitted through the specimen; the image is magnified and
focused onto an imaging device, such as a fluorescent screen, on a
layer of photographic film, or to be detected by a sensor such as
a CCD camera.
TEMs are capable of imaging at a significantly higher
resolution than light microscopes, owing to the small de Broglie
wavelength of electrons. This enables the instrument to be able to
examine fine detail - even as small as a single column of atoms,
which is tens of thousands times smaller than the smallest
resolvable object in a light microscope. TEM forms a major
analysis method in a range of scientific fields, in both physical
and biological sciences. TEMs find application in cancer research,
virology, materials science as well as pollution and semiconductor
research.
Scanning tunneling microscopy (STM) is a powerful technique
for viewing surfaces at the atomic level. STM probes the density
of states of a material using tunneling current. For STM, good
resolution is considered to be 0.1 nm lateral resolution and 0.01
nm depth resolution. The STM can be used not only in ultra high
vacuum but also in air and various other liquid or gas ambients,
and at temperatures ranging from near zero kelvin to a few hundred
degrees Celsius. The STM is based on the concept of quantum
tunnelling. When a conducting tip is brought very near to a
metallic or semiconducting surface, a bias between the two can
allow electrons to tunnel through the vacuum between them. For low
voltages, this tunneling current is a function of the local
density of states (LDOS) at the Fermi level, Ef, of the sample.
Variations in current as the probe passes over the surface are
translated into an image. STM can be a challenging technique, as
it requires extremely clean surfaces and sharp tips.
X-ray scattering techniques are a family of analytical
techniques which reveal information about the crystallographic
structure, chemical composition, and physical properties of
materials and thin films. These techniques are based on observing
the scattered intensity of an X-ray beam hitting a sample as a
function of incident and scattered angle, polarization, and
wavelength or energy.
But all these methods are destructive, very time consuming or
nearly impossible to locate such small regions of interest. The
edge scattering would also prevent clear observation in some
techniques, such as STM. The increasing interests in graphene
demand a fast and non-destructive method to determine the
chirality of edges and the crystal orientation of graphene sheet.
Raman spectroscopy is a proper candidate for this method. As
one of the most commonly used techniques to characterize carbon
related materials, Raman spectroscopy plays a very important role
in acquiring information on the physical, chemical and even
electronic properties of graphene and graphene based devices. It
helps to determine the edge chirality, hence the crystal
orientation of graphene using the difference in intensity of the
disorder-induced D band on the different chiralities of edges
(stronger in armchair edges and weaker in zigzag edges). This
provides an easy and nondestructive method to identify the edge
chirality of graphene, which would help to speed up the practical
applications of graphene nanoelectronic devices, such as GNR.
References
[1] Y. M. You, Z. H. Ni, T. Yu, Z. X. Shen, "Edge chirality
determination of graphene by Raman spectroscopy",
http://arxiv.org/pdf/0810.4981
[2] Kyle A. Ritter, Joseph W. Lyding, "The influence of edge
structure on the electronic properties of graphene quantum dots
and nanoribbons", Nature Materials, 8 (3), (2009)
[3] C. Lee et al. "Measurement of the Elastic Properties and
Intrinsic Strength of Monolayer Graphene". Science, 321- 385,
(2008).
[4] M.Y. Han, B. Ćzyilmaz, Y. Zhang, and P. Kim "Energy Band-Gap
Engi-neering of Graphene Nanoribbons", Phys. Rev. Lett. 98,
206-805, (2007).

Impact of carbon nanotubes on Pakistan economy
Saleem Ullah
Carbon nano tubes are of great interest for researchers
nowadays .The advancement in the carbon nano tubes is going to
play a key role in the economy of every country. I would like to
discuss about the impact of carbon nano tubes on the Pakistan
economy in future. The Pakistan main exports are based on textile
products about 68% which play a key role in Pakistan economy. The
trend is going in textile these days as the consumers demand more
light and good quality material generally with natural cotton
fibers, polyester or some other man made product is mixed in order
to improve the strength and properties. ' one plus one can become
eleven also and need not necessarily be two always,' as a famous
phrase. Recently due to the discovery of worlds hardest plastic
nano- composite material which is created by reinforcing ordinary
plastic with diamonds which are invisible to naked eye, a sheet of
layered carbon and tiny carbon cylinders. Strengthening a common
polymer, polyvinyl alcohol. With nano-diamond, a new age material
called graphene (one atom thick carbon honeycomb sheet) and carbon
nano tube has produced this material with extra hardness and
stiffness. By using this material with natural cotton the
properties can be increased many folds and it can bring a
revolution in the textile world. By using this the Pakistani
textile can get a leading position in the textile world and the
economy will boost exponentially.
The mechanical properties like hardness and stiffness (after
moulding) improved by as much as 400 per cent compared to those
obtained with single reinforcements,' By exploiting this property
the Pakistani sports industry which is the 2nd big export
industry can become the best in the world as the Pakistani sports
products are well known all over the world. A simple recent
example is of hockey stick which is made by using nano carbon
tubes in composite which is of great interest these days all over
the world. Despite its hardness the material is extremely
light-weight. Despite being the world's hardest plastic
nano-composite, the reinforcement material constitutes only one
per cent (by weight) of the composite. To create a similar
material conventionally, 50-60 per cent glass was required as the
reinforcing agent and the resultant composite could not be molded.
Besides excellent mechanical properties, the reinforced polymer
also shows semi-conducting behavior, which could also be
exploited. However, researchers are yet to analyze the new
material's toughness and ductility, without which the extent of
practical application could not be decided.
The other big export products of Pakistan are ceramics
products. A ceramic material reinforced with carbon nano tubes has
been made by materials scientists. The new material is far tougher
than conventional ceramics, conducts electricity and can both
conduct heat and act as a thermal barrier, depending on the
orientation of the nano tubes. The researchers mixed powdered
alumina (aluminum oxide) with 5 to 10 percent carbon nano tubes
and a further 5 percent finely milled niobium. Carbon nano tubes
are sheets of carbon atoms rolled up into tiny hollow cylinders.
With diameters measured in nanometers -- billionths of an inch --
they have unusual structural and conducting properties. The
material shows electrical conductivity ten trillion times greater
than pure alumina, and seven times that of previous ceramics made
with nano tubes. It also has interesting thermal properties,
conducting heat in one direction, along the alignment of the nano
tubes, but reflecting heat at right angles to the nano tubes,
making it an attractive material for thermal barrier coatings.
Carbon nano tubes can also be exploited in defense sector. The
composite material made from nano carbon tubes having a great
mechanical strength can be used in airoplanes,missiles,bullet
proof jackets for army and helmets which will be lighter and more
strengths .In short using the carbon nano tubes Pakistani economy
can boost exponentially and have a positive impact on inhabitants
lives.

Progress in the synthesis of carbon fullerenes and nanotubes
Peerapong Yotprayoonsak
Fullerenes
Fullerenes are the only soluble form of carbon. Some
scientists applied them for technological tasks, others for the
synthesis of new materials. Fullerenes would arouse more interest,
if they were cheaper and more available. Thus, obtaining an
effective technique of fullerene synthesis remains an actual task.
At present the arc method of carbon transformation from graphite
to fullerenes is considered to be more effective [1]. There are
several techniques to carry out with arc method. Some research
group operates in high pressure condition with various gases. On
the other hand, some scientists make it in low pressure with
proper gases or even in vacuum. The schematics of different
pressure conditions are shown as Fig. 1(a) and 1(b) [2, 3].
| (a) |  | (b) |  |
Fig.1 Schematics diagram of
CNT formation apparatus by the arc-discharge method (a) in high
pressure [2] and (b) in low pressure [3].
In addition, the laser vaporization method shown in Fig.2,
which had been originally used as a source of clusters and
ultrafine particles, was developed for fullerene and CNT
production by Smalleys group [3].
 Fig.2 Schematics diagram of
the laser-furnace apparatus [3].
Carbon Nanotubes
In the same way as what happened with fullerene, after carbon
nanotubes were discovered in this world, most scientists have been
interested in this nanostructure until nowadays due to its miracle
properties provided to many of applications not only in physics
and chemistry but also in biology even in medical science.
However, there are still a few drawbacks of the synthesis
technique being solved by scientists such as the high cost, low
uniformity and productivity. However, there are at least 3
techniques to fabricate carbon nanotubes namely arc discharge,
laser ablation and chemical vapor deposition (CVD). Both arc
discharge and laser ablation methods can be simply described by
Fig.1 and Fig.2. Differently from both ways, by adding the
catalysts in the system, one can get carbon nanotubes as well.
This is so-called CVD method yielding the most productive so far.
The common instrument is shown in Fig.3 [4].

Fig.3 Schematics of a CVD deposition oven [4].
At present, the worlds longest carbon nanotube arrays carried
out by CVD technique have been done by researchers at the
University of Cincinnati [5].They are slightly less than 2
centimeters long and each nanotube is 900,000 times longer than
its diameter shown in Fig.4.

Fig.4 The longest carbon nanotube arrays in the
world [6].
On the other hand, the length of individual carbon nanotube
has been produced beyond 2cm long. The reports of the length
comparing with various years and places are shown in Fig.5 (a) and
(b) [7].
| (a) |  | (b) |  |
Fig.5 (a) Electrically
contacted individual SWNT length vs. year (b) Individual SWNT
length vs. year [7].
References
[1] G.N. Churilov, Synthesis of fullerenes and other nanomaterials
in arc Discharge, Invited Lectures.
[2]
http://nobelprize.org/nobel_prizes/chemistry/laureates/1996/illpres/carbon.html
[3] Yoshinori Ando, Xinluo Zhao, Toshiki Sugai, and Mukul Kumar,
Growing Carbon Nanotubes, materialstoday October 2004.
[4] http://ipn2.epfl.ch/CHBU/NTproduction1.htm
[5] http://www.uc.edu/News/NR.aspx?ID=5700
[6] http://www.nsf.gov/news/news_images.jsp?cntn_id=108992&org=NSF
[7] P.J. Burke, C. Rutherglen, Z. Yu, Single-Walled Carbon
Nanotubes: Applications in High Frequency Electronics,
International Journal of High Speed Electronics and Systems Vol.
16, No. 4 (2006) 977-999.

Distinguishing valuable
nanostructures from worthless junk: benefits and limitations of
experimental techniques Elina Yushchenko
A nanostructure is a device of the order of a nanometer in its
smallest dimension. One nanometer is about a few atoms thick, or,
a hundred thousand times thinner than human hair. Existing
commercial devices, such as the Pentium Processors, contain
submicron structures, which are about 100 times larger than
nanostructures linearly, and about 10,000 times larger in area. We
can put more components that are much faster and more energy
efficient in a device of the same size. It is said to be the whole
world [1].
The technical difficulty of fabrication increases, making the
process less reliable. What's worst, since we also want to put
more components in the same device, the probability of getting a
fault component in a device increases dramatically. These are
experimental and technical challenges.
There are several experimental techniques which can be used to
produce nanostructures: Methods for Bulk Synthesis of Carbon,
Pulsed Laser Vaporization, High-pressure CO conversion and
Chemical Vapor Deposition. All of these methods are commonly used
in manufacturing nanotubes, nanofiber, nanoflake and so on.
Experimental Techniques
The arc-evaporation method, which perhaps produces the best
quality nanotubes, involves passing a current of about 50 amps
between two graphite electrodes in an atmosphere of helium. This
causes the graphite to vaporise, some of it condensing on the
walls of the reaction vessel and some of it on the cathode. It is
the deposit on the cathode which contains the carbon nanotubes.
Single-walled nanotubes are produced when Co and Ni or some other
metal is added to the anode. Carbon nanotubes can also be made by
passing a carbon-containing gas, such as a hydrocarbon, over a
catalyst. The catalyst consists of nano-sized particles of metal,
usually Fe, Co or Ni. These particles catalyse the breakdown of
the gaseous molecules into carbon, and a tube then begins to grow
with a metal particle at the tip. It was shown in 1996 that
single-walled nanotubes can also be produced catalytically. The
perfection of carbon nanotubes produced in this way has generally
been poorer than those made by arc-evaporation, but great
improvements in the technique have been made in recent years. The
big advantage of catalytic synthesis over arc-evaporation is that
it can be scaled up for volume production. The third important
method for making carbon nanotubes involves using a powerful laser
to vaporise a metal-graphite target. This can be used to produce
single-walled tubes with high yield [5].
Benefits and limitations
There are lots of advantages of these productions methods,
nevertheless we should mention the limitation of some experimental
techniques. Methods for Bulk Synthesis of Carbon Nanotubes are in
no way limited to supercapacitors, in which nanotubes provide a
combination of high electrical conductivity and high surface area;
field emitters, which require ordered arrays of nanotubes; and
additives to plastics and rubbers that improve their electrical
and thermal conductivity [3].
Isolated SWNT s (single-walled nanotubes) grown on quartz or
silicon subtracts cannot be readily studied using high-resolution
TEM. The sample must be dispersed, or grown, on a conducting
surface to be examined. However, these limitations are not
mentioned to downplay the importance of imaging methods.
Current use and application of nanotubes has mostly been
limited to the use of bulk nanotubes, which is a mass of rather
unorganized fragments of nanotubes. Bulk nanotube materials may
never achieve a tensile strength similar to that of individual
tubes, but such composites may nevertheless yield strengths
sufficient for many applications. Bulk carbon nanotubes have
already been used as composite fibers in polymers to improve the
mechanical, thermal and electrical properties of the bulk product.
Easton-Bell Sports, Inc. have been in partnership with Zyvex,
using CNT technology in a number of their bicycle components -
including flat and riser handlebars, cranks, forks, seatposts,
stems and aero bars.
So, benefits of these techniques are that they are supported by
big companies and technologies are well-known.
References
[1] Y. Zhu, C. Ke and H. D. Espinosa, Experimental Techniques
for the Mechanical Characterization of One-Dimensional
Nanostructures // Experimental Mechanics, Vol. 47, No. 1
[2] M.J.M. Daenen, R. de Fouw, B. Hamers, P.G.A. Janssen, K.
Schouteden, M.A.J. Veld (ST), Wondrous World of Carbon Nanotubes,
URL: http://students.chem.tue.nl/ifp03/default.htm
[3] Yury Gogotsi, Methods for Bulk Synthesis of Carbon Nanotubes,
United States Patent Application 20080219913
[4] M. Meyyappan, Carbon nanotubes, 2004
[5] Peter Harris, Carbon nanotube science and technology, URL:
http://www.personal.reading.ac.uk/~scsharip/tubes.htm

|